J.ophthalmol.(Ukraine).2019;2:28-32.
|
http://doi.org/10.31288/oftalmolzh201922832 Received: 19 November 2018; Published-online: 24 April 2019 Value of neurohumoral dysfunction in the pathogenesis of traumatic optic neuropathy N.M. Moyseyenko, Cand Sc (Med) Ivano-Frankivsk National Medical University; Ivano-Frankivsk (Ukraine) E-mail: natalymoyseenko@gmail.com TO CITE THIS ARTICLE: Moyseyenko NM. Value of neurohumoral dysfunction in the pathogenesis of traumatic optic neuropathy. J.ophthalmol.(Ukraine).2019;2:28-32. http://doi.org/10.31288/oftalmolzh201922832 Background: It is not known in which way neurohumoral properties of the hypothalamus undergo changes due to traumatic optic nerve neuropathy (TON). Purpose: To investigate the value of neurohumoral dysfunction in the pathogenesis of TON. Materials and Methods: Trauma was induced in the right orbital optic nerve in adult rabbits (n = 30, the experimental group). Controls were 30 intact rabbits. Semi-fine and ultra-fine sections were cut, and electron microscopy and morphometry of the right intracranial optic nerve and suprachiasmic hypothalamus nucleus was performed 30 days after traumatic event. Results: Traumatic injury to the orbital optic nerve in rabbits caused reactive edema and destructive changes in the intracranial optic nerve and suprachiasmic hypothalamus nucleus. Decreased volumetric density of neurosecretory granules and increased numbers of pyknomorphic cells at the final stages of their life cycle result in decreased steroid production. This, in turn, promotes inflammatory reactive optic nerve lesions. Conclusion: Neurohumoral dysfunction is an important mechanism of the pathogenesis of traumatic optic neuropathy, and, if corrected, will improve treatment outcomes. Keywords: traumatic optic nerve neuropathy, neurohumoral dysfunction, suprachiasmic hypothalamus nucleus, neurosecretory granules
Introduction Traumatic optic neuropathy (TON) is known to be a disease of numerous etiologies. Supporters of the metabolic pathogenesis theory for TON believe that imbalanced ionic homeostasis, glutamate cytotoxicity, apoptosis of neuronal apoptosis, lipid degradation and inflammatory and immune reactions are secondary mechanisms of damage in this disease [1]. Others have reported that neurochemical factors induced toxic and pro-inflammatory substances (such as prostaglandins [2]), oxidative reactions, chemokines and pro-inflammatory cytokines which destructed the blood brain barrier, causing neural tissue swelling [3-5]. Pasisnichenko [6] has found that the hypothalamus controls and integrates all visceral functions of the body, as well as endocrinous regulation mechanisms with nerve regulation mechanisms. In addition, the hypothalamus is the centre of the autonomic nervous system, which has a regulating effect on the function of cerebral cortex and the structures inferior to it. A decreased functional activity of the anterior hypothalamus (where the suprachiasmal nucleus resides) in the patient who sustained severe craniocerebral trauma has been reported by Klingbeil [7] as early as 1985. Craniocerebral trauma is the most common cause of TON and frequently accompanied by changes in neuropeptide activity [8, 9]. In addition, corticotropin deficiency has been found in 39% of patients with hypopituitarism due to head trauma [10]. Traumatic brain injury can lead to endocrine dysfunction in up to 68% of head-injured patients [11]. It is not, however, known in which way neurohumoral properties of the hypothalamus undergo changes due to traumatic optic nerve damage, and whether these changes affect the development of TON. The purpose of the study was to investigate the value of neurohumoral dysfunction in the pathogenesis of traumatic optic neuropathy. Materials and Methods The experimental study was conducted at the vivarium of the Ivano-Frankivsk National Medical University. Trauma was induced in the right orbital optic nerve in adult Chinchilla rabbits (n = 30, the experimental group) [12]. Controls were 30 intact rabbits. Animals were euthanized using a guillotine 30 days after traumatic event. Semi-fine and ultra-fine sections were cut, and morphological analysis (electron microscopy and morphometry) of the right intracranial optic nerve and suprachiasmic hypothalamus nucleus was conducted at the Electronic Microscopy Laboratory of the University’s Human Anatomy Department. Peripheral serum levels of hydrocortisone and adrenocorticotropic hormone (ACTH) were determined one month after traumatic event. Results and Discussion At day 30 after traumatic event, light microscopy revealed marked swelling and destructive changes in the myelin sheath of myelinated optic nerve fibers (MONFs), with reduced number and size of axons. In addition, in some of these fibers, no axons were found, and the myelin sheath spread over practically the whole area of the fiber (Fig. 1). There were individual MONFs with signs of anisochromia, partial loss of their normal structure, and damage of the myelin sheath. Some MONFs, however, were found to preserve their structure. Moreover, the endoneural connective tissue was edematous (most pronounced in the subperineural area and around microvessels).
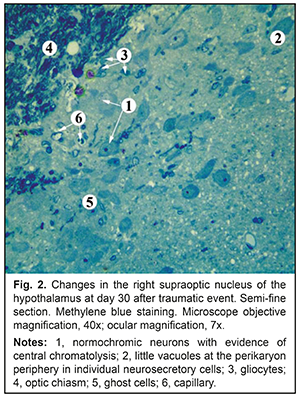
There was no significant difference in the cross-sectional area of axons in the right optic nerve between experimental animals and controls (0.92±0.61 mm2 vs 0.96±0.72 mm2, р=0.93457). There was, however, a significant difference in the cross-sectional area of MONFs (3.09±1.98 mm2 vs 2.25±1.53 mm2, р=0. 026336), with a g index for experimental animals as low as 0.29±0.08 compared to 0.42±0.06 for controls, indicating myelin sheath swelling. At day 30 after traumatic event, in the right supraoptic nucleus of the hypothalamus in experimental animals, there were increased numbers of neurosecretory cells, with evidence of peripheral chromatolysis, hypochromic nuclei and ghost cells (Fig. 2). Little vacuoles were seen at the perikaryon periphery in individual neurosecretory cells. Most neurosecretory cells demonstrated karyopyknosis, and some of them, karyolysis.
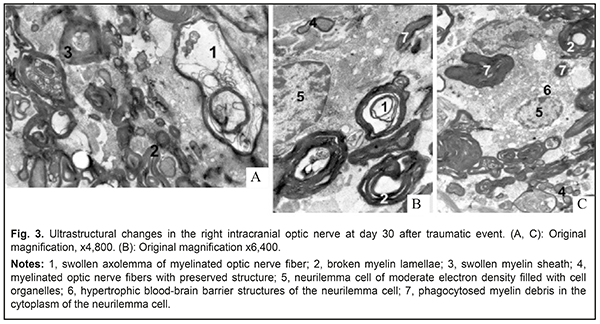
There was a significant difference in the cross-sectional area of perikaryons of the right supraoptic nucleus between experimental animals and controls (276.59±38.02 μm2 vs 244.12±35.50, р=0.0428). There was, however, no significant difference in the cross-sectional area of the nucleus (71.93±15.67 μm2 vs 78.58±14.30, р=0.2515). The above increase in the cross-sectional area of perikaryons, in combination with a decrease in the nucleocytoplasmic index from 0.47±0.07 to 0.35±0.07 (р=0.0001), indicated not an increased functional activity of neurosecretory cells, but swelling of and destructive changes in nuclei and cells. These things are a sign of reactive changes in the suprachiasmatic nucleus of the hypothalamus in experimental animals one month after traumatic event. Ultrastructural microscopy revealed destructive changes in MONFs in the right intracranial optic nerve, which manifested either as swollen and thickened myelin sheath (Fig. 3a) or internal myelin layers exhibiting protrusions of different of shapes and height (Fig. 3a, b). Large-diameter MONFs with a thin myelin sheath were also noted (Fig. 3a).
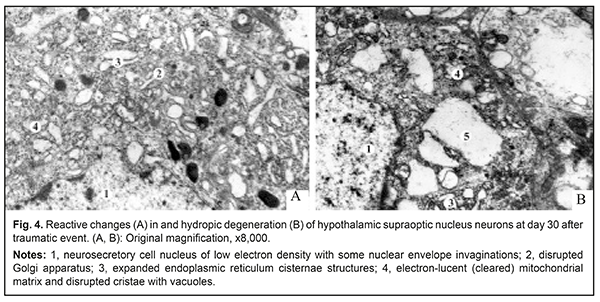
The axoplasm of axons in these fibers was electron transparent, and there was lysis of neurofibrils and mitochondria, indicating local axon swelling with decreased axon flow. In most MONFs, there were numerous protrusions made up of internal myelin lamellae and appearing as dendritic, fan-shaped or fiber-shaped structures. The axoplasm of axons in these MONFs exhibited numerous vacuoles of different diameters and swollen mitochondria with cleared matrix and disrupted cristae. Some authors [13, 14] believe that such axonal changes are evidence of impaired axon flow. In areas of myelin sheath loss, axons had irregular contours, there were areas of axonal dilation and/or stenosis, alternating hyperchromic and hypochromic areas, and individual axons were fragmented. Endoneural swelling began to subside and demonstrated fine-grained myelin breakdown products. At this time point of the experiment, neurilemma cells were noted to increase in numbers and size. The cytoplasm of these cells showed homogeneous masses of myelin sheath breakdown products and hypertrophic blood-brain barrier structures (BBBS) (Fig. 3a). In addition, degenerating axon debris was lying freely among intensely stained myelin masses. The cytoplasm of some neurilemma cells had moderate electron density and was filled with cell organelles (Fig. 3a, b), whereas other neurilemma cells showed expanded perinuclear space, and their cytoplasm contained individual vacuoles (Fig. 3c). Such changes in neurilemma cell structure were indicative of their high functional activity which was associated both with phagic activity in damaged myelinated nerve fibers (MNFs) and with regeneration of MNFs. Thus, newly formed MNFs were seen beside the MNFs undergoing degenerative changes. The former were small diameter, round-shaped fibers with normal myelin sheath and well-defined mesaxon (Fig. 3c). The axoplasm of their axons was of moderate electron optic density and contained young mitochondria and microtubules. Endoneural swelling was noted to contain numerous fibroblasts and collagen fibrils. There were MNFs with evidence of demyelination, manifested as myelin sheath disorganization and myelin debris lying freely in cytoplasm of neurilemma cells. Some authors [15, 16] interpret these changes as mesaxon destruction under conditions of impaired energy and protein metabolism. In addition, neurilemma cells reduce their metabolic load through transformation of myelin lamellae into cytoplasmic inclusions of myelin fragments, which may be considered as adaptive response aimed at improving conditions for regeneration of nerve fibers. The above changes in optic nerve structure take place in the presence of reconfiguration of the supraoptic hypothalamus nucleus. In the ultrastuctural study, the cytoplasm of most light neurosecretory cells of the supraoptic hypothalamus nucleus demonstrated expanded endoplasmic reticulum cisternae structures, electron-lucent (cleared) mitochondrial matrix, disrupted cristae with vacuoles, increased numbers of primary and secondary lysosomes, and disrupted Golgi apparatus (Fig. 4). Neurosecretory cell nuclei were of low electron density and contained some nuclear envelope invaginations.
There were individual light neurosecretory cells with hydropic degeneration phenomena. Volumetric density of neurosecretory granules in these neurons was significantly decreased compared to controls (2.24±0.19% vs 6.95±0.36, respectively, р=0.0002). Minor vacuoles, lysosomes, increased numbers of expanded endoplasmic reticulum cisternae structures, multivesicular bodies and individual neurosecretory granules were seen in the neuroplasma of dark neurosecretory cells. The nuclei of these cells were electron dense and had one to two nucleoli. Volumetric density of neurosecretory granules in dark neurosecretory cells was significantly decreased compared to controls (1.14±0.04% vs 3.56±0.12, respectively, р=0.0002). Among dark neurosecretory cells, there were cells undergoing destructive changes and having no neurosecretory granules, but containing lysosomes. Some authors attribute such hypothalamic neurons to pyknomorphic cells that are at the final stages of their life cycle [17]. These highly osmophylic cells undergo total shrinkage. Chromophile and pyknomorphic cells are mentioned specifically among dark neurons by others [18-20], like us. The former are characterized both by high RNA levels in the nucleus and nucleoli and by the absence of irreversible destructive changes, from which the authors conclude that chromophile neurons are more functionally active, whereas pyknomorphic neurons are those that are at the final stages of their life cycle. The above morphological changes in the structure of the hypothalamus were seen in the presence of changes in blood hormone levels. In experimental animals, peripheral serum levels of hydrocortisone and ACTH were lower than in controls (11.79±0.12 μg/dl vs 92.31±3.26 μg/dl, p<0.05, and 6.91±0.09 pg/dl vs 11.64±0.43 pg/dl, p<0.05, respectively). Therefore, we found that traumatic injury to the orbital optic nerve in rabbits caused the reactive edema and destructive changes in the intracranial optic nerve and suprachiasmatic hypothalamus nucleus. Decreased volumetric density of neurosecretory granules and increased numbers of pyknomorphic cells that are at the final stages of their life cycle result in decreased steroid production. This, in turn, promotes inflammatory reactive optic nerve lesions that obviously require correction, which may have a neuroprotective effect for restoration of the optic nerve. Conclusion Neurohumoral dysfunction is an important mechanism of the pathogenesis of traumatic optic neuropathy, and, if corrected, will improve treatment outcomes. References
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|