J.ophthalmol.(Ukraine).2019;2:22-27.
|
http://doi.org/10.31288/oftalmolzh201922227 Received: 05 February 2019; Published-online: 24 April 2019 Features of the course and treatment of JIA-associated uveitis M.V. Panchenko,1 Dr Sc (Med); N.S. Shevchenko,2,3 Dr Sc (Med); M.V. Demianenko,3 MD; Honchar O.M., 1 Cand Sc (Med); L.G. Avilova,1 MD 1 Kharkiv National Medical University 2 Karazin Kharkiv National University 3 Pediatric and Adolescent Health Institute of the NAMS of Ukraine; Kharkiv (Ukraine) E-mail: panchenko0802@gmail.com TO CITE THIS ARTICLE: Panchenko MV, Shevchenko NS, Demianenko MV, Honchar OM, Avilova LG. Features of the course and treatment of JIA-associated uveitis. J.ophthalmol.(Ukraine).2019;2:22-7. http://doi.org/10.31288/oftalmolzh201922227 Purpose: To investigate the features of the clinical course of and the state of eye care for patients with juvenile idiopathic arthritis (JIA)-associated uveitis (JIA-U). Materials and Methods: We analyzed the results of monitoring 121 patients aged 7 to 18 years with JIA. Twenty one (17.35%) of these had uveitis. Results: In 17 (80.95%) of the examined JIA-U patients, JIA onset age was younger than 6 years, and mean JIA onset age was significantly younger than in JIA patients without uveitis (2.89±0.26 years vs 6.43±0.4 years, р<0.05). Most (85.7%) JIA-U patients were asymptomatic. Chronic keratic precipitates and posterior synechiae were found in 38.09% and 42.85%, respectively, of cases. Uveal cataract was the most common complication (57.14%), followed by corneal degeneration (33.33%), macular edema (28.57%), ocular hypertension/ secondary glaucoma (19.05%), optic nerve edema (14.28%), hypotony (14.28%), vitreous fibrosis (9.52%), and retinal detachment (4.76%). The frequency of administration of biologics was 57.14%. The mean time from disease onset to administration of biologics in our JIA-U patients (2.67±0.79 years) was 2.5 times less than the value for JIA-U patients from all over Ukraine (6.9 years), indicating that biologics were timely administered. Conclusion: Patients with the condition are commonly asymptomatic and have frequent ocular complications, with uveal cataract being the most common, followed by corneal degeneration and macular edema. Keywords: uveitis, juvenile idiopathic arthritis
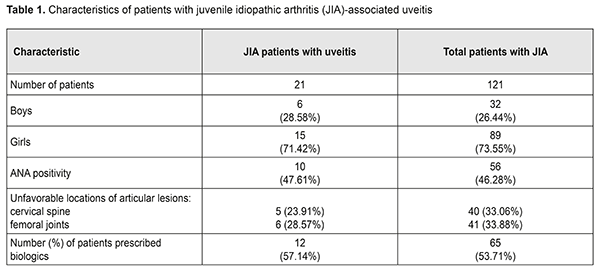
Introduction Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease of childhood, with JIA-associated uveitis (JIA-U) its most frequent extra-articular manifestation [1, 2]. Pediatric uveitis is a topic of special interest not only because of the unique diagnostic and therapeutic challenges but also because of the lifetime burden of vision loss if the problem is not adequately treated, as well as the economic and psychological toll on the family and society as a whole [3]. Uveitis is the fourth most common cause of blindness in developed countries [4]. The causes of uveitis are numerous (approximately 50), and include HLA-B27 positivity, tubulointerstitial nephritis, Behçet’s disease, sarcoidosis, and viral, parasitic and bacterial factors [5, 6]. Smith et al [7] found that idiopathic uveitis, JIA-U, and pars planitis were the leading diagnoses in pediatric uveitis. JIA-associated uveitis accounts for 20% to 25% [7, 8] and even as much as 39.4% [9] of cases with pediatric uveitis. Major risk factors for the development of uveitis in JIA are oligoarticular pattern of arthritis, an age at onset of arthritis of less than seven years of age, and antinuclear antibody (ANA) positivity [8, 10]. It has been found that uveitis appeared simultaneous with or within the first 6 months of arthritis onset in almost half (48%) of cases of JIA [11]. Approximately 12% to 38% of patients with JIA develop uveitis in seven years following onset of arthritis [8, 12]. In the initial stages of mild to moderate inflammation, uveitis is not timely diagnosed [8]. However, the underlying pathogenic mechanisms remain unclear, particularly regarding interplay between genetic and environmental factors. Work is in progress on updating novel (a) risk markers for pediatric inflammatory uveitis and severe disease course, (b) approaches to the treatment of resistant disease, and (c) criteria for the measurement of imaging endpoints and assessment of therapy efficacy. There are several new genetic markers, biomarkers and clinical factors that may influence a child’s uveitis disease course [13]. In a large cohort of patients with JIA [11], anterior uveitis was the most common anatomic type of uveitis (83%), whereas intermediate uveitis (9%), posterior uveitis (1%) and pan-uveitis (7%) were less frequent.. In a study by Cosickic et al [12], 78% of children with JIA-U had bilateral uveitis. This condition is usually asymptomatic [10, 12, 14], and thus screening for JIA-U in at-risk patients is paramount. Early detection and treatment aims to stop inflammation and prevent the development of complications leading to visual loss. Complications of JIA-U include cataracts, glaucoma, optic disc edema, retinal detachment, epiretinal membranes, amblyopia, linear keratopathy, hypotony, etc [10, 15-17]. In 30% to 50% of children with JIA-associated uveitis structural complications are present at diagnosis. Of these, about 50% to 78% will eventually complications [8, 9, 18, 19]. Uveitis treatment strategies for patients with JIA are still widely discussed. Glucocorticosteroids are probably the most widespread treatment, but, since they have substantial side effects and low efficacy in achieving disease remission, resorting to immunosuppressive treatments is a frequent practice [3, 4]. There is an increasing amount of evidence supporting early use of immunosuppressive treatments to reduce the use of topical and systemic glucocorticosteroids. Findings of some studies suggest that although giving oral GCS in sufficiently low doses (i.e., ≤7.5 mg/day) for long periods is safe, it is of low efficacy; therefore, immunosuppressive agents should be used as a part of early treatment for uveitis [5]. This includes disease-modifying antirheumatic drug (DMARD) therapy (principally methotrexate) and biological agents targeted at particular cytokines (most commonly, monoclonal antibodies targeted against TNF-α). Adalimumab is used in the management of non-infectious, intermediate, posterior and pan-uveitis uveitides and considered more efficacious than other biological agents [5, 20]. A study on the analysis from the UK Childhood Arthritis Prospective Study (CAPS) [21] has reported that the percentage of patients prescribed biologics for JIA increased to 16%-38%. Evidence has been reported [20, 22, 23] supporting the use of biologics (in particular, adalimumab) in patients with early onset, chronic anterior uveitis, which was in most cases associated with JIA, in case of inadequate response to topical therapy and methotrexate, with improved long-term control of disease. In addition, the incidence of visual impairment and ocular complications was lower and improvement was observed earlier than in other studies [16, 22], and special attention was given to long-term treatment outcomes. The SITE study [19] has concluded that increasing uveitis activity was associated with increased risk of vision loss and use of immunosuppressive drugs was associated with reduced risk of vision loss suggesting that control of inflammation and use of immunosuppression may be critical aspects in improving the outcomes of patients with JIA-related uveitis. Severe intraocular inflammation, chronicity of uveitis at a young age, late first visit to an ophthalmologist, ocular complications, and posterior uveitis are among the factors causing blindness in children with uveitis [15]. That is why observation of patients with JIA-associated uveitis should be guided by the current Single Hub and Access point for pediatric rheumatology in Europe (SHARE) consensus-based recommendations for diagnosis, screening, disease activity measurement, and treatment [24]. The purpose of the study was to investigate the features of the clinical course of and the state of eye care for patients with JIA-associated uveitis based on the data from the Pediatric and Adolescent Health Institute of the NAMS of Ukraine. Materials and Methods We analyzed the results of monitoring 121 patients (age, 7 to 18 years) with polyarticular (76.03%) or oligoarticular (23.96%) juvenile idiopathic arthritis, disease duration longer than 5 years, and clinical and radiographic evidence of arthritis. These patients have been supervised and treated at the Pediatric and Adolescent Health Institute of the NAMS of Ukraine. Of these, 21 (17.35%) patients had uveitis. The percentage of girls was higher than that of boys (71.42% vs 27.58%, р <0.001). The mean age was 9.95±0.19 years (124.76±2.31 months). The mean JIA onset age was 5.21±0.18 years (63.53±2.19 months), and mean disease duration was 4.88±0.19 years (58.52 ±2.27 months). JIA was diagnosed as per the Unified Clinical Protocol, and all children received treatment with methotrexate, folic acid and non-steroidal anti-inflammatory drugs at relevant doses (Order of Ministry of Health of Ukraine No 832 dated October 22, 2012, On Approval of the Unified Clinical Protocol of Medical Care in Juvenile Idiopathic Arthritis). In addition, data from the Ukrainian Register of Patients with JIA (Ukrainian Pediatric Rheumatologist Association, //ura.medireg.com.ua) were subjected to analysis. Results and Discussion Girl patients outnumbered boy patients (Table 1), which agrees with the literature [25, 19].
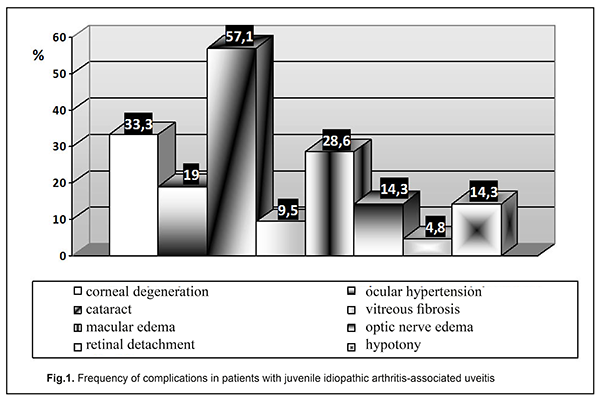
In 17 (80.95%) of the examined JIA patients with uveitis, JIA onset age was younger than 6 years, and mean JIA onset age was significantly younger than in JIA patients without uveitis (2.89±0.26 years vs 6.43±0.4 years, р<0.05), which agrees with other studies [25]. In the CARRA registry [26], of the 3967 patients with JIA, 11.6% had uveitis. In addition, systemic, polyarticular, oligoarticular, psoriatic and enthesitis-related subtypes accounted for 1%, 24.9%, 57.1%, 6.9% and 7.6%, respectively, of the JIA-U patients. In March, 2018, the Ukrainian Registry of Patients with JIA involved 437 patients with JIA from 33 regional centers; of these patients 14.43% had uveitis. In addition, polyarticular, oligoarticular, enthesitis-related subtypes accounted for 55.56%, 23.81% and 6.35%, respectively, of the JIA-U patients. Moreover, of the JIA-U patients, 55.6% experienced the onset of uveitis before the onset of arthritis, 19.05% developed uveitis after the onset of arthritis, and 25.4% were unaware whether the onset of uveitis preceded or followed the onset of arthritis. In children who developed uveitis before the onset of arthritis, the maximum time between the onset of uveitis and the onset of arthritis was 8.67 years. In children in whom uveitis was diagnosed after the development of arthritis, the interval ranged from one month (31 days) to 5.25 years. Our findings are generally in line with those from the CARRA Registry [26] and the Ukrainian Registry of Patients with JIA. The overall prevalence of uveitis in our JIA cohort (17.35%) was somewhat higher than those reported from the above registries. In addition, polyarticular (RF-negative only) and oligoarticular subtypes accounted for 56.87% and 43.13%, respectively, of our JIA-U patients. However, in most of our JIA-U patients, the onset of ocular involvement was not recorded, and ocular involvement was diagnosed only after visiting a cardio-rheumatologist. Only in 3 (14.29%) patients, it was an ophthalmologist who diagnosed uveitis as a JIA-associated condition, which was followed by administration of relevant medications. Most (85.7%) JIA-U patients were asymptomatic. Chronic keratic precipitates and posterior synechiae were found in 38.09% and 42.85%, respectively, of cases. In addition, posterior synechiae were found in 42.85% of cases, which is in agreement with the data reported by others (29% - 52% [19, 25, 27]). The frequency of complications of uveitis is presented in Figure 1.
Uveal cataract was the most common complication (57.14%), followed by corneal degeneration (33.33%), macular edema (28.57%), ocular hypertension/ secondary glaucoma (19.05%), optic nerve edema (14.28%), hypotony (14.28%), vitreous fibrosis (9.52%), and retinal detachment (4.76%). This is in agreement with the findings by others who found that cataract, corneal degeneration, and macular edema were the most common complications in patients with JIA-U [1, 25, 27, 28]. In the study by Angeles-Han et al [25], cataract, corneal degeneration, glaucoma and macular edema were found in 31%, 25%, 17%, and 15%, respectively, of the 52 patients with JIA-U. Gregory et al [19] examined 327 children with JIA-U, and revealed corneal degeneration, macular edema, hypotony and ocular hypertension in 31.4%, 5.4%, 4.5%, and 18%, respectively, of these patients. In addition, 40.1% of these patients underwent cataract or glaucoma surgery. Frequencies of corneal degeneration and glaucoma (33.33% and 19.05%, respectively) in JIA-U patients of the current study are generally in line with those reported by others. However, the frequencies of macular edema (28.57%), optic nerve edema (14.28%) and hypotony (14.28%) are substantially higher than those reported by others. A study by Skarin et al [28] found that frequencies of cataract and uveitic glaucoma in patients with JIA-U increased with uveitis duration, and cataract was present in 51%, whereas uveitic glaucoma was present in 22% at 24 years, which agrees with our findings (cataract was present in 57.14%, and ocular hypertension and glaucoma were present in 19.05% of our patients with JIA-U). However, a study by Stroh et al [29] reported that the frequency of ocular hypertension or secondary glaucoma in patients with JIA-associated uveitis can be as high as 40%, which is two times as high as our value. Therefore, children with JIA-U are commonly asymptomatic, and the disease is characterized by frequent complications. In the current study, the mean JIA onset age was 5.21±0.18 years (63.53±2.19 months), and the mean age at treatment onset was 6.92±0.19 years (86.02 ±2.44 months). In addition, the mean interval between age at diagnosis and initiation of methotrexate therapy, the gold standard for treating children with JIA, was 23.31±1.36 months. That is, administration of correct treatment occurred on average 1.7 years later than it should have, and the treatment lag was much longer than the window of opportunity [30, 31]. The JIA treatment protocol requires that methotrexate is administered from diagnosis at relevant doses in combination with local therapy. Poor treatment efficacy in the presence of threatening factors in the form of HLA-B27 or ANA positivity, or unfavorable locations of articular lesions, requires biologics administration 6-12 months after the onset of the pathological process [32]. In the current study, we found that, in children with JIA-U, the frequency of administration of biologics was 57.14%, with a mean time from disease onset to administration of biologics of 2.67±0.79 years. In addition, it should be noted that there were isolated cases of administration of adalimumab for persistent inflammation 13 years from the onset of JIA and 9 years after diagnosis of uveitis. According to the Ukrainian registry data, in Ukraine, 44 children with JIA-U (69.84%) are administered biologics. This percentage is the highest among JIA subtypes, and the children with JIA-U administered biologics account for 15.77% of all children with JIA administered biologics. However, with regard to the Ukrainian registry, the mean time from disease onset to administration of biologics was 6.88 years. That is, biologics were administered too late after disease onset, at the time when irreversible ocular changes have already occurred. It should be noted that, the frequency of administration of biologics (57.1%) in our patients was in line with the value from CARRA Registry (56%) [33], although somewhat lower than the value from the Ukrainian registry (69.8%). In addition, the mean time from disease onset to administration of biologics in our patients with JIA-U (2.7 years) was 2.5 times less than the value for patients with JIA-U from all over Ukraine (6.9 years), evidencing that biologics were timely administered. Conclusion Therefore, patients with JIA-U are asymptomatic, which makes diagnosis difficult, and may develop severe ocular complications. Our JIA-U patients were found to have frequent complications, with uveal cataract being the most common (57.14%), followed by corneal degeneration (33.33%) and macular edema (28.57%). In our cohort of patients with JIA-U, frequencies of macular edema (28.57%) and optic nerve edema (14.28%) were substantially higher than those reported by others, possibly due to the uveitis course in these patients, and a wider administration of biologics may reduce the values. This study not only highlighted the features of the course of JIA-associated uveitis, it also indicated the need for cooperation between specialties in the management of the condition and timely prescription of appropriate therapy. References 1.Clarke SL, Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol Online J. 2016 Apr;14(1):27. 2.Sen ES, Dick AD, Ramanan AV. Uveitis associated with juvenile idiopathic arthritis. Nat Rev Rheumatol. 2015;11:338–48. 3.Wentworth BA, Freitas-Neto CA, Foster CS. Management of pediatric uveitis. F1000Prime Rep. 2014 Jun;6:41. 4.Mérida S, Palacios E, Navea A, Bosch-Morell F. New Immunosuppressive Therapie sin Uveitis Treatment. Int J Mol Sci. 2015 Aug;16(8):18778-95. 5.Jabs DA. Immunosuppression for the Uveitides. Ophthalmology. 2018 Feb;125(2):193-202. 6.Tugal-Tutkun І. Pediatric Uveitis. J Ophthalmic Vis Res. 2011;6(4):259-69. 7.Smith JA, Mackensen F, Sen HN et al. Epidemiology and course of disease in childhood uveitis. Ophthalmologу. 2009 Aug;116(8):1544-51. 8.Ramanan AV, Dick AD, Benton D et al., SYCAMORE Trial Management Group. A randomised controlled trial of the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate for the treatment of juvenile idiopathic arthritis associated uveitis (SYCAMORE Trial). Trials. 2014 Jan;15:14. 9.Ftrrara M, Eggenschwiler L, Stephenson A et al. The Challenge of Pediatric Uveitis: Tetriary Referral Center Experience in the United States. Ocul Immunol Inflamm. 2018 Jan;15:1-8. 10.Foeldvari I, Walscheid K, Heiligenhaus A. Uveitis in juvenile idiopathic arthritis. Z Rheumatol. 2017 Oct;76(8):664-72. 11.Heiligenhaus A, Niewerth M, Ganser G et al. Prevalence and complications of uveitis in juvenile idiopathiс arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatology. 2007; 46:1015–1019. 12.Cosickic A, Halilbasic M, Selimovic A, Avdagic H. Uveitis Associated with Juvenile Idiopathic Arthritis, our Observations. Med Arch. 2017 Feb; 71(1): 52-5. 13.Angeles-Han ST, Rabinovich CE. Uveitis in children. Curr Opin Rheumatol. 2016 Sep;28(5):544-9. 14.Heiligenhaus A, Minden K, Foll D, Pleyer U. Uveitis in juvenile idiopathic arthritis. Dtsch Arztebl Int 2015; 112: 92–100. 15.Boer J, Wulffraat N, Rothova A. Visual loss in uveitis of childhood. Br J Ophthalmol. 2003 Jul;87(7):879-84. 16.Cann M., Ramanan AV., Crawford A. et al. Outcomes of non-infectious Paediatric uveitis in the era of biologic therapy. Pediatr Rheumatol Online J. 2018 Aug;16(1):51. 17.Hersh AO, Cope S, Bohnsack JF et al. Use of Immunosuppressive Medications for Treatment of Pediatric Intermediate Uveitis. Ocul Immunol Inflamm. 2018;26(4):642-50. 18.Dajee KP, Rossen JL, Bratton ML et al. A 10-year review of pediatric uveitis at a Hispanic dominated tertiary pediatric ophthalmic clinic. Clin Ophthalmol. 2016 Aug;10:1607-12. 19.Gregory AC, Kaçmaz RO, Foster CS et al. Systemic Immunosuppressive Therapy for Eye Diseases Cohort Study Research Group. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the Systemic Immunosuppressive Therapy for Eye Diseases Study. Ophthalmology. 2013 Jan;120(1):186-92. 20.Ramanan AV, Dick AD, Jones AP et al. SYCAMORE Study Group. Adalimumab plus Methotrexate for Uveitis in Juvenile Idiopathic Arthritis. N Engl J Med. 2017 Apr;376(17):1637-46. 21.Davies R, Carrasco R, Foster HE et al. Treatment prescribing patterns in patients with juvenile idiopathic arthritis (JIA): Analysis from the UK Childhood Arthritis Prospective Study (CAPS). Semin Arthritis Rheum. 2016 Oct; 46(2): 190–195. 22.Cecchin V, Zannin ME, Ferrari D et al. Longterm Safety and Efficacy of Adalimumab and Infliximab for Uveitis Associated with Juvenile Idiopathic Arthritis. The Journal of Rheumatology. 2018 Aug; 45 (8) 1167-72; 23.Quartier P, Baptiste A, Despert V. et al. ADJUVITE Study Group. ADJUVITE: a double-blind, randomised, placebo-controlled trial of adalimumab in early onset, chronic, juvenile idiopathic arthritis-associated anterior uveitis. Ann Rheum Dis. 2018 Jul;77(7):1003-11. 24.Constantin T, Foeldvari I, Anton J et al. Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis. 2018 Aug;77(8):1107-17. 25.Angeles-Han ST, McCracken C, Yeh S, et al. Characteristics of a cohort of children with Juvenile Idiopathic Arthritis and JIA-associated Uveitis. Pediatr Rheumatol Online J. 2015 Jun;13:19. 26.Angeles-Han ST, Pelajo CF, Vogler LB et al. CARRA Registry Investigators. Risk markers of juvenile idiopathic arthritis-associated uveitis in the Childhood Arthritisand Rheumatology Research Alliance. J Rheumatol. 2013 Dec;40(12):2088-96. 27.Paroli MP, Abbouda A, Restivo L et al. Juvenile idiopathic arthritis-associated uveitis at an Italian tertiary referral center: clinical features and complications. Ocul Immunol Inflamm. 2015 Feb;23(1):74-81. 28.Skarin A, Elborgh R, Edlund E, Bengtsson-Stigmar E. Long-term follow-up of patients with uveitis associated with juvenile idiopathic arthritis: a cohort study. Ocul Immunol Inflamm. 2009;17(2):104–8. 29.Stroh IG, Moradi A, Burkholder BM, et al. Occurrence of and Risk Factors for Ocular Hypertension and Secondary Glaucoma in Juvenile Idiopathic Arthritis-associated Uveitis. Ocul Immunol Inflamm. 2017 Aug;25(4):503-512. 30.Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003;48(7):1771–4. 31.Nigrovic PA. Review: is there a window of opportunity for treatment of systemic juvenile idiopathic arthritis? Arthritis Rheumatol. 2014;66(6):1405–13. 32.Ravelli A, Consolaro A, Horneff G, et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2018 Jun; 77(6): 819-828. 33.Henderson LA, Zurakowski D, Angeles-Han ST et al. Medication use in juvenile uveitis patients enrolled in the Childhood Arthritis and Rheumatology Research Alliance Registry. Pediatr Rheumatol Online J. 2016; 14: 9.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|