J.ophthalmol.(Ukraine).2019;2:14-21.
|
http://doi.org/10.31288/oftalmolzh201921421 Received: 12 March 2019; Published-online: 24 April 2019 Prevention of macular edema and secondary degeneration of the macula and posterior pole in anterior uveitis N.V. Konovalova, Dr. Sc. (Med.); N.I.Khramenko, Cand. Sc. (Med.); O.V. Guzun, Cand. Sc. (Med.) SI “The Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine”, Odessa (Ukraine) E-mail: kvnkonovalova@gmail.com TO CITE THIS ARTICLE: Konovalova NV, Khramenko NI, Guzun OV. Prevention of macular edema and secondary degeneration of the macula and posterior pole in anterior uveitis. J.ophthalmol.(Ukraine).2019;2:14-21. http://doi.org/10.31288/oftalmolzh201921421 Background. Endogenous uveitis is an inflammatory disease of the uvea, making up to 30% in the ophthalmology overall structure according to various authors. Uveitis is among current socially important issues in ophthalmology due to significant incidence rates mainly in people of young and working age; visual impairment and blindness occur in 17.7%-19.2-35% of patients. Purpose. To improve the treatment efficacy in patients with anterior uveitis using Indocollyre® electrophoresis and Indocollyre® instillations in order to prevent macular edema and secondary degeneration of the macular and the anterior pole. Material and Methods. Fifty-four patients (54 eyes) with anterior uveitis were followed up. All patients were performed biomicroscopy, ophthalmoscopy, intraocular pressure measurements, perimetry, and ophthalmic rheography using a parameter of pulse volume, the Rheographic Quotient (RQ) (‰) (ReoCom, Ukraine). Optical coherent tomography (OCT) visualized structural changes not only in the sensory retina but in the choroid, which makes it possible to assess the inflammation process in the eye and to early diagnose and to prevent potential complications. Fluorescein angiography (FAG) was performed in order to assess the pigment epithelium, sensory epithelium, and vessels as well as to reveal drusen and the accumulation of lipofuscin in the retina. Results. OCT findings give the evidence of the presence of macular edema if the retina thickness is increased in the fovea (>240 µm). This is evidenced by the FAG findings and revealed difference in the rheographic quotient value of more than 64% in 21 patients, which made it possible to predict the risk of a dry form of macular dystrophy in these patients. Timely prescription of Indocollyre® electrophoresis and instillations advanced macular edema resolution, which contributed to significant improvement of visual acuity in the iridocyclitis patients, as much as twice, in both groups. The mean treatment course in an acute process was 10±1.8 days. Conclusion. It is reasonable to use Indocollyre® electrophoresis and instillations to prevent such anterior uveitis complications as macular edema and secondary macular degeneration. Keywords: anterior uveitis, macular edema, degeneration of the macula and posterior pole, ophthalmic rheography, electrophoresis, Indocollyre
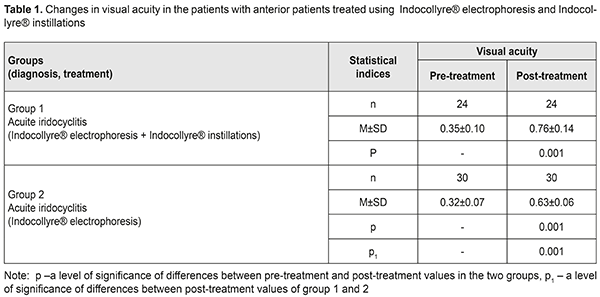
Background Uveitis is the inflammation of the uvea and refers to multifactorial diseases. In a broader concept, uveitis is a synonym to “intraocular inflammation” [14]. Endogenous uveitis are among current socially important issues in ophthalmology due to a significant incidence, variety of etiological factors, pathogenesis complicacy, and high rates of complications, leading to visual impairment in 17.7% and monocular blindness in 19.2-35% of patients [4]. Numerous epidemiologic studies, performed in different countries, have shown that the most common type of uveitis is anterior uveitis (37-62%), followed by posterior uveitis (9-38%) and panuveitis (7-38%); isolated intermediate uveitis is diagnosed less frequently, in 4 to 17% [9,11]. In most developed countries, uveitis takes 10 to 15% in the structure of blindness and visual impairment causes [19, 13]. According to B. Trusko, in the USA, up to 30 thousand new cases of uveitis-associated blindness are registered annually, which takes the fifth or sixth place among all blindness causes [24]. Chronic uveitis is more common than acute ones, compiling 50-60% of all uveitis. Non-granulomatous uveitis is more prevalent than granulomatous one, especially in anterior uveitis. Non-infectious uveitis is more frequent than infectious one, particularly in anterior uveitis and panuveitis. In uveitis etiopathogenesis, the main role belongs to the development of immune responses to damaged layers of the eye and infectious agent invasion. Etiological factors of uveitis development are multivariate. According to D. Denislam (1994) [2], any microorganism, able to induce an inflammation process, can be a cause of uveitis. A high rate of uveitis is associated with extensive branching of blood vessels and, in this regard, slow blood flow in the uvea; this is favorable for microbes, viruses, and other pathogenic agents, which can cause inflammation under specific conditions, to stay in the uvea. They are herpes virus, tuberculosis, toxoplasmosis, and syphilis agents that affect the uvea most frequently. Endogenous uveitis in focal infection (chronic tonsillitis, sinusitis, dental disorders) is often induced by staphylococcus and streptococcus. Recently, a higher priority in uveitis etiology has been given to genetic factors. Some people, genetically predisposed to autoimmune diseases, have a higher risk of uveitis. These diseases include ankylosing spondylitis, ulcerative colitis, Crohn's disease, and psoriasis. Anterior uveitis is often associated with HLA-B27 antigen; Behçet's disease is associated with HLA-B51; Vogt-Koyangi-Harada syndrome is associated with HTA-DR4; and sarcoidosis, sympathetic ophthalmia, and peripheral uveitis are associated with HLA-DR15. Uveitis combined with systemic changes make up approximately 25-30% of all uveitis [5, 10]. Not all antigen carriers pointed suffer from uveitis or an antigen-associated systemic disease. However, not only genetic predisposition but an environmental factor, acting as a trigger for pathologic reactions, is required for a disease to develop. This inflammation-inducing trigger in genetically predisposed persons is understudied. According to one of uveitis pathogenesis concepts, an inflammatory process in the uvea is considered as an interaction of factors, including genetic predisposition, general and local sensitization, disorders of the blood-aqueous barrier, and reinvasion of an antigen into the eye. The latter factor can be both direct entry and that influenced by general diseases and stressful situations, when general and local immunity is reduced, which contributes to antigen penetrating into the eye from extraocular inflammatory tissues. The role of infections in uveitis etiology is undeniable; however, they are given a part of a trigger for the process development while the higher priority is given to immune mechanisms. Iridocyclitis can be caused by systemic diseases such as toxoplasmosis, viral infection, a rheumatic disease, tuberculosis, syphilis, and ocular trauma. The role of various infectious lesions in immune-compromised patients with HIV infection, diabetes, and Hepatitis C is increasing. Another cause of uveitis can be viral lesions of the uvea, syphilis, leprosy, and brucellosis. In some conditions like keratouveitis, sclerouveitis, and retinal vasculitis, inflammation can be spread to or occur simultaneously in other ocular structures [14]. Despite a lot of up-to-date diagnostic tests aimed to define uveitis etiology, it is impossible to determine a cause of uveitis in 38-40% of patients. These cases refer to idiopathic uveitis [18, 15]. OCT can be used to reveal and measure focal morphological changes, retinal thickness, and nerve fibre layer. Macular edema, affecting outer and inner retinal layers, occurs in 26-32% of acute anterior uveitis and in 64% of posterior uveitis, which leads to irreversible blindness in 8.5% [7, 11]. Early diagnosis and treatment of edema and the dystrophic process is crucial to protect the retina. One of the most common structural complications of uveitis is macular edema which is the most frequent cause of visual impairment. Damage to the blood-retinal barrier by cytokines leads to fluid leakage into intercellular space and fluid accumulation in the outer plexiform and outer nuclear layers around the fovea. Two mechanisms of edema development, inflammatory and hypoxic, have been proposed in order to explain the occurrence of cystoid macular edema in such a variety of ocular conditions. An inflammation theory can explain chronic uveitis-associated edema while a hypoxic mechanism of occlusion, due to a food chain of the choriocapillary layer with outer retinal layers, can explain conditions associated with vascular pathology [16, 22, 23]. These two mechanisms intersect in every particular case of cystoid macular edema. This can be seen as fluorescein penetrating in angiograms and as the increased retinal thickness in OCT images. These two tests demonstrate different manifestations of inflammation and since the retinal thickness is more towards correlating with visual acuity, OCT is more often used to evaluate the changes in the disease. Persistent macular edema can lead to irreversible destruction of neuronal connections in the retina, gliosis, and atrophy, resulting in permanent visual loss [23, 6]. At the present time, the gold standard for the treatment of macular edema is intravitreal injections. However, corticosteroid injections are not always safe; there can often be such complications as increased intraocular pressure, complicated cataract, endophthalmitis. A search for new, safer, and, at the same time, effective treatments for macular edema is a relevant objective in uveitis treatment. Inflammation-involved prostaglandins, in a very short time, induce the damage to the blood-aqueous barrier since they affect the permeability of vessel walls. Due to permeability disruption, protein and inflammatory cells penetrate into the anterior chamber of the eye [13, 25]. There, these cells get into the intraocular fluid and, therefore, increase the intraocular pressure. Prostaglandin-induced disruption of vessel permeability leads to accumulation of liquid in the retinal layers of the posterior pole of the eye and to elevated intraocular pressure. If these changes occur for a long time, they cause functional visual loss [28]. In general, non-steroid anti-inflammatory drugs (NSAIDs) are effective for the prevention and treatment of cystoid macular edema since they inhibit the synthesis of prostaglandins [17]. NSAIDs as prostaglandin synthesis inhibitors block cyclooxygenase. NSAIDs have become an alternative to corticosteroids due to the fact that their anti-inflammatory action is effective in the same way while the rate of undesirable effect is very low [12, 20]. Indocollyre® eye drops stabilize the blood-aqueous barrier. Indocollyre® eye drops contain low-concentrated indometacin and have a strong anti-inflammatory action in the damaged area and are optimally tolerated by ocular tissues. The main characteristics of the drug are anti-inflammatory, anti-miosis, and analgesic. Indocollyre® eye drops provide optimal penetration of indometacin through the cornea and, therefore, to the target tissues. The anti-miosis mechanism of indometacin is based on strong inhibiting the activity of the substance P, which, being a mediator, has a special action in the anterior chamber aqueous humour and is inhibited by indometacin [26]. Thus, uveitis is a common inflammatory eye disease which mainly affects people of the working age. It is rather difficult to specify a cause of inflammation and a multi-stage examination, excluding systemic, local, and autoimmune diseases, is required. In most cases, the disease is characterized by the recurrent or chronic course with both eyes affected and risk of visual impairment and blindness in 10-15% of patients. Treatment and prevention of endogenous uveitis complications is still challenging due to a lack of pathogenically- substantiated drugs with a selective mechanism of action on ophthalmic inflammation agents. In this regard, improvement of treatment efficacy for these patients remains one of the primary objectives in the modern ophthalmology. One of the most relevant directions is the development of complex etiopathogenetic approaches, focused on the exaggeration of the anti-microbial and anti-inflammatory action in therapy, as well as on prevention of severe complications, reduction of side effects of a general and local character. Of particular significance in prevention and treatment for ocular inflammations is the development of new effective methods for interruption of processes leading to inflammation and developing macular edema and secondary degeneration of the macula and the anterior pole. Purpose. To improve the treatment efficacy in patients with anterior uveitis using electrophoresis and Indocollyre® instillations in order to prevent macular edema and secondary degeneration of the macula and the anterior pole. Material and Methods Fifty-four patients (54 eyes) with anterior uveitis were treated in Department for Inflammatory Pathology at SI “Filatov Institute of Eye Diseases and Tissue Therapy”. The patients’ age averaged (32.7±1.4) years old. The patients underwent an antibacterial desensitizing therapy and pupil massages. The patients received instillations and electrophoresis using Indocollyre®, which contains low-concentrated indometacin (0.1%). The drug regimen varied so the patients were divided into two groups: group 1, the patients received Indocollyre electrophoresis and Indocollyre instillation, thrice a day, for 10 days in addition to a standard anti-inflammatory therapy; group 2, the patients received Indocollyre electrophoresis for 10 days in addition to a standard anti-inflammatory therapy. The mean course of treatment was (10±1.8) days. Routine physiotherapeutic technique was used. During transorbital electrophoresis, an active electrode in the form of a tray was poured with 2-3 ml of 2% calcium chloride solution and then with 1 ml of Indocollyre. An anode is positive. The current is increased in stages from 0.3-0.5-0.8 mA to 1 mA, duration of the procedure 3-5-8-10 minutes. An indifferent electrode with hydrophilic coating was in the neck area. During intranasal electrophoresis, an active electrode was in the form of nasal turunda. An anode is positive. The current is increased in stages from 0.3-0.5-0.8 mA to 1 mA, duration of the procedure 3-5-10 minutes. All patients were performed biomicroscopy, ophthalmoscopy, intraocular pressure measurements, perimetry, and ophthalmic rheography using a parameter of pulse volume, the Rheographic Quotient (RQ) (‰) (ReoCom, Ukraine). Optical coherent tomography (OCT) visualized structural changes not only in the sensory retina but in the choroid, which makes it possible to assess the inflammation process in the eye and to early diagnose and to prevent potential complications. Fluorescein angiography (FAG) was performed in order to assess the pigment epithelium, sensory epithelium, and vessels as well as to reveal drusen and the accumulation of lipofuscin in the retina. The data obtained were statistically processed using Statistica 7.0 software. The Student's t-test was used for pair-wise comparison of the two groups and preliminary assessment of normality of distribution [1]. Results and Discussion Complex anti-inflammatory therapy using Indocollyre® for the patients with iridocyclitis resulted in the improvement of the clinical picture and visual function in all patients. Table 1 demonstrates the data on the changes in visual acuity in the patients of two groups.
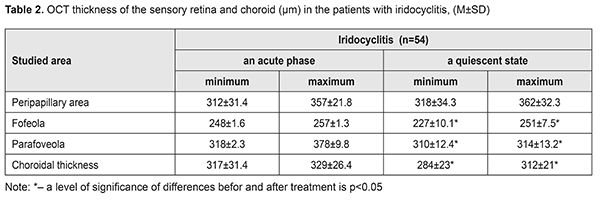
Thus, visual acuity improved significantly, almost twice, in both groups. However, visual acuity increased to 0.76 (SD 0.14) in group 1, where the patients additionally received Indocollyre® instillations, which is higher by 17% (р<0.05) as compared to group 2. OCT was used to determine morphologic parameters of the sensory retina and the choroid at different phases of the process in the patients with anterior uveitis (Table 2). The mean thickness of the sensory retina in norm varies from (197.4±1.5) μm to (252.1±12.2) μm in the fovea, from (312.5±55.3) μm to (377.4μm±19.9) μm in the parafovea, and from (347.7±61.8) μm to (440.8±64.9) μm in the peripapillary retina.
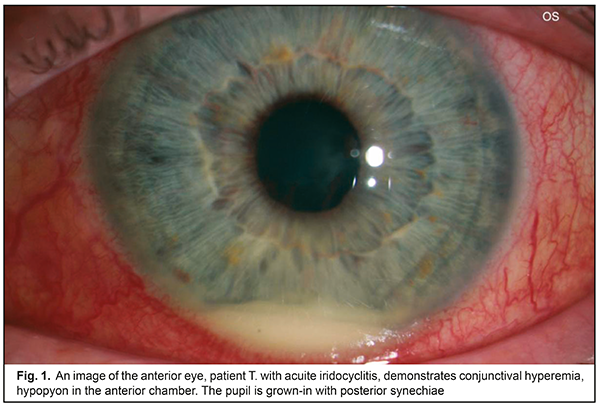
Clinical signs of macular edema were loss of foveal reflex, pathological flares, retinal folds, grayish color, and thickened retina. No signs of cystoid macular edema (fluid-filled vesicles in the center) were revealed. Morphological parameters of the patients were compared before and after treatment. After treatment, both minimal and maximal values of the retinal thickness decreased by 8.5% and 2.5% for the fovea and the parafovea, respectively (р<0.05). The mean choroidal thickness varied from (271.7 ± 24.3) to (315.5 ± 22.3) μm. Increased choroidal thickness, up to 317 - 329 μm, was noted in the fovea of the patients with acute iridocyclitis. On completion of an anti-inflammatory treatment, this parameter decreased by 10%-5.5% (р<0.05). Initial dystrophic changes are associated with damaged differentiation of retinal layers. The foveola is characterized by a specific architectonics of retinal layers. Towards the center of the foveola, the thickness of outer nucleus layer increases while inner layers of sensory epithelium gradually disappear. Various sources are involved in the nutrition of the outer and inner retina: six retinal layers are supplied with blood by retinal capillaries from the central retinal artery while outer retinal layers are supplied by the choroidal blood vessels. In the presence of macular edema, fluid is accumulated in the outer retinal layers. On completion of in-hospital treatment, the patients of both groups received Indocollyre® instillations, thrice a day for 1 month. 9 patients from group 1 and 12 patients from group 2 were examined at a follow-up visit at month 1. A thinning of the retina, most pronounced in the fovea – by 38.8 – 41.2% (р=0.0001), was noted in two patients of group 1 and three patients of group 2, which was indicative of an early stage of a dystrophic process. These patients continued the course for complication and prevention by supplementing the inflammation-involved tissues with required nutrients (Ocuvite® Complete, 1 capsule a day for 6 months). A clinical case The treatment performed resulted in managing the inflammation in the anterior eye; macular edema resolved; at follow-up visits, single dystrophic foci were observed in the paramacular retina. All 54 patients with recurrent uveitis were performed a complex clinical and functional examination of the visual system using the ophthalmic rheography. The rheography findings, characterizing haemodynamics in the uveitis patients, showed that, in the patients with acute uveitis, Rheographic Quotient (RQ) in the affected eye was higher by 27% (р=0.05) than RQ in the fellow eye, (4.92±0.31)‰ (coefficient of variation (CV), 21.1) vs. (3.88±0.40)‰, (CV, 28%), respectively. It should be noted that an increase in pulse volume in the affected eye as compared to norm (RQ = (3.50±0.11) ‰) was 40% (р<0.05). Pulse volume in the fellow eye also tended to increase. The difference in RQ (∆RQ) between an affected and fellow eye (21 patients) was 1.21±0.50‰. A high variability of difference in pulse volume between paired eyes should be noted: the bilateral pulse volume difference ranged from 0.2 to 3.3‰. In 33 patients with managed inflammation, RQ in the affected eye was (2.39±0.23)‰ (CV, 33%) and did not differ significantly from RQ in the fellow eye, which was equal to (2.65±0.5)‰. A decrease of this parameter in a quiescent state compared to acute inflammation was 52% (р<0.05); moreover, pulse volume in the fellow eye also decreased by 31% (р<0.05). Pulse volume in a quiescent state was decreased by averagely 30% in both affected and fellow eyes as compared to norm. In 21 patients (38.8%), the difference between Rheographic Quotient values was more than 64 %, which made it possible to predict the risk of a dry form of macular dystrophy in these patients. A method to predict a development of macular degeneration in the patients with chronic uveitis (iridocyclitis) included rheography with rheographic quotients measured and compared in paired eyes; in the RQ difference is 63% and more, macular dystrophy was predicted. There was detected a dependence of blood circulation disorders in the vessels of the ciliary body on the acuity of the process and the period of uveitis remission, which is of practical importance for choosing an optimal therapy and for controlling the disease during remission. Revealed blood circulation disorders in the ciliary vessels and the changes in hydrodynamics in uveitis help determine the characteristics of a pathologic process in the eye and use a comprehensive therapy to avoid complications and recurrences. Electrophoresis and instillations with Indocollyre® were proved to be effective for prevention of such anterior uveitis complications as macular edema, and, as a consequence, macular degeneration. In intercurrent period, a decrease in pulse volume by 30% in both affected and fellow eyes requires an anti-ischemic therapy and nutritional supplement (Ocuvite, 1 capsule a day for six months). Conclusions OCT findings give the evidence of the presence of macular edema if the retina thickness is increased in the fovea (>240 µm). Acute iridocyclitis is also accompanied by the increased choroidal thickness in the fovea, to 317 - 329 μm, and by pulse volume, increased by 40% compared to norm. Fluorescein leakage in FAG can serve as an indirect predictor in uveal maculae edema treatment. Timely prescription of Indocollyre® electrophoresis and instillations advanced macular edema resolution, which contributed to significant improvement of visual acuity in the iridocyclitis patients as much as twice, in both group. It also helped decrease the retinal and choroidal thicknesses. The mean treatment course in an acute process was 10±1.8 days. All patients were prescribed Ocuvite, 1 capsule a day for six months, which made it possible to stabilize the process and preserve visual acuity. To conclude, firstly, endogenous uveitis is among relevant and socially important issues in ophthalmology and is characterized by high rates of complications leading to visual impairment and blindness in 19.2%-35%. Secondly, a combined anti-inflammatory treatment using Indocollyre® electrophoresis and instillations in the patients with anterior uveitis resulted in significantly improved visual acuity in both groups, as much as twice; the retinal thickness decreased in the fovea and parafovea by 8.5% and 2.5%, respectively; the choroidal thickness in the fovea was normalized. Finally, it is reasonable to use Indocollyre® electrophoresis and instillations to prevent such anterior uveitis complications as macular edema and secondary macular degeneration. In addition, all patients can be prescribed Ocuvite, 1 capsule a day for six months, which contribute to stabilizing the process and preserving visual acuity.
References 1.Glanz S. [Biomedical statistics]: translation from English. M.: Practice; 1998. 459. Russian. 2.Zhaboiedov GD, Skrypnyk RL, Baran TV. [Eye Diseases]. K.:VSV Meditsyna;2011.99p. Ukrainian. 3.Katargina LA, Arkhipov. [Uveitis: a pathogenic immunosuppressive therapy]. Tver; 2003. 99p. Russian. 4.Katargina LA, Slepova OS, Krichevskaia GN. [Immune diagnosis and immunotropic treatment for endogenous uveitis]. [Proceedings of Actual problem of Ophthalmology]. M.;2003: 370-1. Russian. 5.Konovalova NA, Ponomareva MN, Gnatenko LE. [Comparative analysis of changes in uveitis incidence]. Meditsinskaia nauka i obrazovaniie Urala. 2015;16(1(81)):92-4. Russian. 6.Konovalova NV, Khramenko NI, Scheibe Abderahim, Ivanitskaya EV, Naritsina NI. [Study of the state of the sensory part of the retina and ocular vascular membrane in patients with uveitis using optic coherent tomography]. Oftalmol Zh. 2014;3:34-41. Russian. 7.Kopaienko AI, Zhaboiedov GD. [State of macular area in the retina based on OCT findings in patients with anterior endogenous uveitis]. Fedorovskiie chteniia. M.; 2009: 76. Russian. 8.Suchina L, Lysenko A, Yulish E. [The optimization of diagnostics and treatment of the chronic recurrent uveitis in children, caused by persistent intracellular infections]. Oftalmologiia. Vostochnaia Evropa. 2014;2(21):20-6. Russian. 9.Anesi SD, Foster CS. Anterior uveitis: etiology and treatment. Advanced Ocular Care. 2011. Feb:32-34. 10.Coscas G, Loewenstein A, Bandello F. Optical Coherence Tomography. ESASO Course Series. 2014;4:26-33. 11.Dayani PN. Posterior uveitis: an overview. Advanced ocular care. 2011; Feb: 32-4. 12.Diestelhorst M, Schmidl B, Konen W, Mester U, Sunderaj P. Efficacy and tolerance of diclofenac sodium 0.1%, flurbiprofen 0.03 % and indomethacin 1.0 % in controlling postoperative inflammation. J Cataract Refract Surg. 1996;22 (Suppl.): 7880793. 13.Durrani OM, Meads CA, Murray PI. Uveitis: a potentionaly blinding disease. Ophthalmologica. 2004;218:223–36. 14.Foster CS, Vitale AT. Diagnosis and treatment of uveitis – Second Ed. Jaypee Brothers Medical Publishers; 2013. 1276 p. 15.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of uveitis study. Ophthalmology. 2004; 111(3):491-500 16.Groot-Mijnes JDF, Visser L, Zuurveen S et al. Identification of new pathogens in the intraocular fluid of patients with uveitis. Am. J. Ophthalmol. 2010;150(5): 628 – 36. 17.Tomkins–Netzer O, Lightman S, Drye L. Outcome of treatment of uveitic macular edema. The multicenter uveitis steroid treatment trial: 2-year results. Ophthalmology. 2015;122:2351–59. 18.Nussenblatt RB, Whitcup SM. Uveitis: fundamental and clinical practice. 4 – th Ed. Elsevier Inc. 2010. 433 p. 19.Rothova A, Suttorp-Schulten MS, Treffers FW, Kijlstra A. Causes and frequency of blindeness in patients with intraocular inflammatory disease. Br. J. Ophthalmol. 1996;80:332–6. 20.Sanders R, McEwen CJ, Haining А. Comparison of prophylactic topical and subconjunctival treatment in cataract surgery. Eye. 1992:105-10. 21.Silverstein A. Changing trends in the etiological diagnosis of uveitis. Documenta Ophthalmologica.1997;94:25-37. 22.Smith JA. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009;8:1544-51. 23.Tomkins–Netzer O, Lightman S, Drye L. Outcome of treatment of uveitic macular edema. The multicenter uveitis steroid treatment trial: 2-year results. Ophthalmology. 2015;122:2351–59. 24.Trusko B, Thort J, Jabs D. The Standardization of Uveitis Nomenclature (SUN) Project. Development of clinical evidence base utilizing informatics tools and techniques. Methods Inf. Med. 2013;7(52 (3)):259–65. 25.Trinquand C, Roux M, Dupin O, Arnaud B. Evaluation of non steroidal anti inflammatory agents in presumed bacterial conjunctivitis: comparison of 0,1 % indomethacin and 0,25% prednisolone in association with 1 % rifamycin. New Trends in Ophthalmology. 1989;X(304):1995. 26.Unger WG. Mediation of the ocular response to injury and irritation: peptides versus prostaglandins. Prog. Clin. Biol. Res. 1989;312: 293. 27.Visser L. Infectious uveitis. New deveIopments in ethiology and pathogenesis. Netherlands, Enschede: Gildeprint Drukkerijen; 2009. 227 p. 28.Weinstein GW. Cataract surgery In: William Tasman (Ed.). Duane’s Clinical Ophthalmology J. B. Lippincott Company. 1990;5(7):39.
This paper has been supported by Bausch Health.
|