J.ophthalmol.(Ukraine).2019;2:55-60.
|
http://doi.org/10.31288/oftalmolzh201925560 Received: 19 December 2019; Published-online: 24 April 2019 Oxidation and peroxidation in the uvea of the rabbit eyes with experimental uveitis and ocular hypertension I.M. Mikheitseva, Dr Sc (Biol); N.V. Bondarenko, Postgraduate Student; S.G. Kolomiichuk, Research Fellow; T.I. Siroshtanenko, Junior Research Fellow Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine; Odessa (Ukraine) E-mail: filatovbiochem@ukr.net TO CITE THIS ARTICLE: Mikheitseva IM, Bondarenko NV, Kolomiichuk SG, Siroshtanenko TI. Oxidation and peroxidation in the uvea of the rabbit eyes with experimental uveitis and ocular hypertension. J.ophthalmol.(Ukraine).2019;2:55-60. http://doi.org/10.31288/oftalmolzh201925560 Background. The role of metabolic changes in the anterior eye tissues in the pathogenesis of uveal inflammation in patients with elevated intraocular pressure has been studied poorly. Of particular interest, in this respect, are free radical mechanisms, which can be a trigger of oxidant stress and cause damage to cell membranes in ocular tissues. Purpose. To study the activity of pro-oxidant enzymes and levels of lipid peroxidation products in the uvea tissues in the rabbits with experimental anterior non-infectious uveitis against the background of ocular hypertension. Material and Methods. Forty-one rabbits were divided into 4 study groups. Group 1: 10 animals with experimental ocular hypertension; group 2: 10 animals with experimental allergic uveitis; group 3: 12 animals with ocular hypertension and allergic uveitis; and group 4: 9 intact animals serving as controls. To simulate ocular hypertension in the groups 1 and 3, the animals were made a single 0.1 ml injection of 0.3% carbomer into the anterior chamber. The tissues of the uvea and the aqueous humour were studied biochemically. We estimated the activity of pro-oxidant enzymes, NADPH oxidase and xanthine oxidase, and the content of lipid peroxides: malonic dialdehyde and diene conjugates. The data of the experimental studies were processed using parametric statistical tests with an SPSS package and Statistica 5.5 Results. The activity of pro-oxidants enzymes was increased in all uvea tissues in all study groups; the maximal NADPH oxidase and xanthine oxidase activity was in the animals with both ocular hypertension and uveitis. The NADPH oxidase and xanthine oxidase activity was increased by 51.1% and 63.9% (р<0.001), respectively, as compared with controls. Lipid peroxidation with accumulation of toxic products in the uvea and aqueous humour was noted both in the ocular hypertension-only and uveitis-only groups; however, the maximal values were in an uveitis model against the background of ocular hypertension, where malonic dialdehyde and diene conjugates were increased, respectively, by 67.0% and 54.3% (р<0.001) in the aqueous humour, and, respectively, by 93.1% and 69.1% (р<0.001) in the uvea tissues, compared with controls. Conclusions. Our findings reveal an important link in the pathogenic action of elevated IOP which burdens inflammation in the uveal tissues through the activation of oxidative and peroxidative processes. In addition, our findings support the assumption that primary high-pressure glaucoma can be a factor which worsens inflammation in the anterior eye. Kewords: ocular hypertension, uveitis, oxidation, lipid peroxidation, malonic dialdehyde, diene conjugates, rabbits
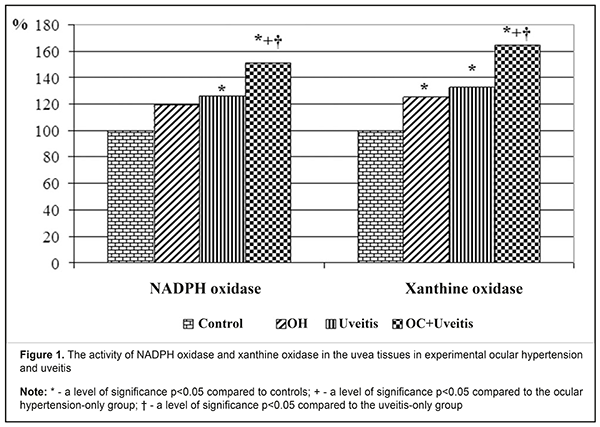
Introduction Primary open angle glaucoma (POAG) is a polyetiologic disease with a threshold effect and which is associated with many risk factors, including intraocular pressure rates, age, hereditary background, diabetes mellitus, etc.. Primary open angle glaucoma development is caused by metabolic and microstructural changes at the cellular level as a consequence of disorders in involutive and biomechanical processes, insufficient vascular autoregulation and insufficient blood flow, increased nerve cell apoptosis, and decreased natural neuroprotection. The disease is also associated with elastotic characteristics of the sclera, vascular dysregulation, age, changes in immune factors, ethnicity, atherosclerosis, and others [1, 2, 3, 4, 5]. Nowadays, studies on immune mechanisms of primary open angle glaucoma have revealed significant cellular and humoral dysimmunity. In the pathogenesis of primary open angle glaucoma, there can be an autoimmune inflammation followed by the destruction of ocular tissues. Antibodies to tissues of the vascular tract, retina, and optic nerve and proinflammatory cytokines have been found in the blood serum and tears of POAG patients. These factors can cause the development of uveitis in POAG [6, 7]. All physiological and pathogenic processes in the body have been proved to be associated, directly or indirectly, with the structure and function of biological cell membranes. These structures are considerably characterized by free radical chain reactions as physiological processes. However, an increased rate of free radical chain reactions is pathology and contributes to the development of various diseases in the body [8, 9, 10, 11, 12]. A pathologically activated free-radical reaction, particularly lipid peroxidation, is known to have a pathogenetic role in the glaucoma development. First of all, free-radical mechanisms are involved in the formation of ocular hydrodynamic disorders and increased intraocular pressure which is one of the major glaucoma symptoms. Pathological changes, associated with activated oxygen and oxygen metabolites, lead to destructive processes in the drainage system of the eye. It is assumed that the aqueous humour outflow can be reduced due to the increased content of “abnormal metabolites” in the humour and their toxic effect. Among those metabolites, in particular, are products of lipid peroxidation. Activation of free radical processes leads to disorders in aqueous humour outflow and damage to the structure and function of the trabecular meshwork, in particular [8, 9, 11, 13, 14]. The role of metabolic changes in the anterior eye tissues in the pathogenesis of uveal inflammation in patients with elevated intraocular pressure has been studied poorly. Of particular interest, in this respect, are free radical mechanisms, which can be a trigger of oxidant stress and cause damage to cell membranes in ocular tissues [14, 15, 16]. In view of this, studies on the pathogenetic role of peroxidation, oxidation and the state of pro-oxidant enzyme system in anterior eye tissues in glaucoma-complicated non-infectious inflammation of the uvea are currently important. The purpose of the present paper was to study the activity of pro-oxidant enzymes and levels of lipid peroxidation products in the uvea tissues in the rabbits with experimental anterior non-infectious uveitis against the background of ocular hypertension. Material and Methods The experiment was conducted on Chinchilla rabbits, weighed approximately 3 kg. The animals were kept under standard vivarium conditions and received food and water ad libitum. The experiment followed the General Ethical Principles of Animal Experiments (Third National Congress on Bioethics, Ukraine, Kyiv, 2007) and European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Strasbourg, 1986). The experiment involved forty-one animals which were divided into 4 study groups. Group 1: 10 animals with experimental ocular hypertension; group 2: 10 animals with experimental allergic uveitis; group 3: 12 animals with ocular hypertension and allergic uveitis; and group 4: 9 intact animals serving as controls. To simulate ocular hypertension in the animals of groups 1 and 3, the animals were made a single 0.1 ml injection of 0.3% carbomer in the anterior chamber [17]. To simulate allergic uveitis, the animals underwent general anaesthesia through 50 mg/kg ketamine administration, following local eye drop instillations of 0.5% procaine hydrochloride. Allergic uveitis was modelled using our method through the administration of bull serum albumin with a dose of 5 mg in previously sensibilized animals (Patent Application No u 201900513, Ukraine. A method of the modelling of non-infectious uveitis against ocular hypertension / I.M. Mikheitseva, S.G. Kolomiichuk, N.V. Bondarenko, T.I. Siroshtanenko). Intraocular pressure (IOP) was measured by a contact Maklakov tonometer using a 7.5 g plunger under local anaesthesia (0.5% proxymetacaine hydrochloride). IOP levels were (13.5±0.6) mmHg in controls; (18.7±1.2) mmHg in the ocular hypertension-only group; (15.3±1.4) mmHg in the anterior uveitis-only group; and (23.4±1.3) mmHg in the ocular hypertension and anterior uveitis group. Biological material for biochemical studies of aqueous humour and uvea tissues was collected 4 weeks after the modelling of ocular hypertension and anterior uveitis. The animals were taken out of the experiment under deep anaesthesia (10% thiopental sodium, 1ml/1kg) by air embolism. The eyes were enucleated on the ice at the temperature of 0ºС-5ºС. The tissues of the uvea and the aqueous humour were studied biochemically; the iris and the ciliary body were used to prepare homogenate using 0.9% sodium chloride with the ratio of 1:9 (weight: volume). The obtained extracts were centrifuged at 5 ºС for 10 minutes with 1000 r.p.m. We estimated the activity of pro-oxidant enzymes: NADPH oxidase [18] and xanthine oxidase [19]; and the content of lipid peroxides: malonic dialdehyde and diene conjugates [20]. The content of metabolites and the activity of enzymes were measured in the supernatant fluid by an SF-26 spectrophotometer and a Specol – 210 spectrocolorimeter. The findings of experimental studies were processed using parametric statistical tests with an SPSS package and Statistica 5.5 [21, 22]. Results and Discussion The present experiment studied, first of all, the activity of enzymes, which produce active forms of oxygen, in the uvea of rabbits with ocular hypertension and uveitis. Figure 1 demonstrates the data on a relative difference in changes in the enzymatic activity in the ocular tissues of the rabbits with experimental ocular hypertension and uveitis.
In the ocular hypertension-only animals, the NADPH oxidase activity was statistically insignificantly increased by 19.6% (р>0.05) in the uvea tissues, (0.110±0.008) nkat/mg protein vs. (0.092±0.006) nkat/mg protein in controls; in the uveitis-only animals, that was increased by 25% (р<0.05) compared with controls and equalled (0.115±0.007) nkat/mg protein. The NADPH-oxidase activity was statistically highly increased in the animals with ocular hypertension and uveitis, by 51.1% (р<0,001) as compared with controls, and equalled (0.139±0.009) nkat/mg protein. This differed by 26.4% (р<0.05) and 20.9% (р<0.05) comparing with, respectively, the ocular hypertension-only and uveitis-only groups. Changes in the xanthine oxidase activity in the uvea tissues in ocular hypertension and uveitis were even more pronounced. Thus, the xanthine oxidase activity was increased in the ocular hypertension-only, uveitis-only, and ocular hypertension and uveitis groups, as compared to controls, by 25.0% (р<0.05), 33.3% (р<0.01), and 63.9% (р<0.01), respectively, and equaled (0.045±0.003) nkat/mg protein, (0.048±0.003) nkat/mg protein, and (0.059±0.004) nkat/mg protein, respectively, vs. (0.036±0.002) nkat/mg protein in controls. When comparing the data of the ocular hypertension and uveitis group with those of the ocular hypertension-only and uveitis-only groups, the xanthine oxidase activity was increased by 31.1% (р<0.01) and 22.9% (р<0.05), respectively. Thus, it can be concluded that the activity of NADPH oxidase and xanthine oxidase in uvea tissues in rabbit eyes is increased both in the presence of elevated intraocular pressure and an allergic uveitis model; moreover, the most pronounced changes are noted in an allergic uveitis model against the background of elevated intraocular pressure. Such enzymes as NADPH oxidase and xanthine oxidase are known to be major in the generation of active oxygen forms, including superoxide radical which can induce free radical oxidation with the formation of organic hydroperoxides. Thus, the increased activity of superoxide-producing enzymes against the background of the depleted enzyme antioxidant system contributes to deep oxidative stress in the anterior eye tissues in uveitis with elevated intraocular pressure. Considering the fact that NADPH oxidase is regulated by compounds which also participate in the pathogenesis of vascular diseases, the role of superoxide-producing enzymes in the genesis of endothelial dysfunction in ocular hypertension and uveitis is not excluded. Afterwards, we evaluated the lipid peroxidation processes in the uvea and aqueous humour of the experimental animals, particularly the accumulation of lipid peroxides: malonic dialdehyde and diene conjugates (Tables 1, 2).
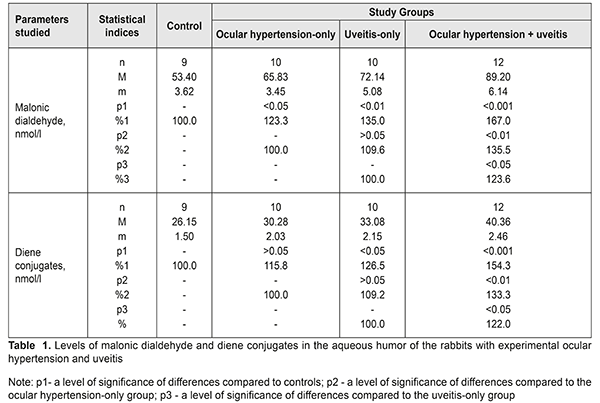
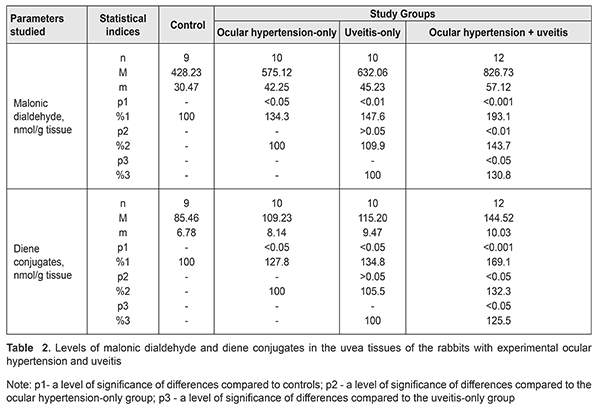
The level of malonic dialdehyde in the aqueous humour was increased by 23.3% (р<0.05), 35,0% (р<0,01), and 67.0% (р<0.001) in the ocular hypertension-only, uveitis-only, and ocular hypertension and uveitis groups, as compared to controls. Comparing the data between study groups showed that malonic dialdehyde in the ocular hypertension and uveitis group was increased by 35.5% (р<0.01) and 23.6% (р<0.05) compared with the ocular hypertension-only and uveitis-only groups, respectively. The level of diene conjugates in the aqueous humour tended to increase in the ocular hypertension-only group and differed insignificantly from the controls, by 15.8% (р>0.05). The diene conjugates level in the uveitis-only group was increased by 26.5% (р<0.05) as compared with controls. The level of diene conjugates in the ocular hypertension and uveitis group was significantly increased by 54.3% (р<0.001) compared with controls and by 33.3% (р<0.05) and 22.0% (р<0.05) compared with the ocular hypertension-only and uveitis-only groups, respectively. Table 2 demonstrates data on levels of malonic dialdehyde and diene conjugates in the uvea tissues of the rabbits with ocular hypertension and uveitis. The levels of malonic dialdehyde were increased by 34.3% (р<0.05) and 47.6% (р<0.01) in the ocular hypertension-only and uveitis-only groups, respectively, compared with controls. Maximal changes in the malonic dialdehyde level were noted in the animals with both ocular hypertension and uveitis. Here, the malonic dialdehyde level increased by 93.1% (р<0.001), 43.7% (р<0.01), and 30.8% (р<0.05) compared with controls, the ocular hypertension-only group, and the uveitis-only group, respectively. Studies on diene conjugates in the uvea of the rabbits showed the increased levels of diene conjugates as compared with controls: by 27.8% (р<0.05) in the ocular hypertension-only group; by 34.8% (р<0.05) in the uveitis-only group; and by 69.1% (р<0.001) in the ocular hypertension and uveitis group. The level of diene conjugates in the ocular hypertension and uveitis group was higher by 32.3% (р<0.05) and 25.5% (р<0.05) as compared with the ocular hypertension-only and uveitis-only groups, respectively. Evaluating the levels of lipid peroxides, it should be noted that the level of end products of lipid oxidation (malonic dialdehyde) was higher than that of intermediate products (diene conjugates) in all study groups, which can be caused by rates of lipid peroxidation of primary lipid peroxides in the given conditions. In addition, the most significant changes in the studied parameters, both in the aqueous humour and the uvea, were in the animals with uveitis against the background of elevated IOP. We believe that this confirms the fact that elevated IOP in a model of uveitis contributes to a significant rise of the pathogenic effect of these metabolites on the anterior eye tissues. The higher level of malonic dialdehyde and diene conjugates in the uvea tissues of rabbits can also be due to the diffusion of lipid peroxidation products from the aqueous humour. This possibility is not excluded since the major aqueous humour outflow occurs through the anterior pathways (in particular, through the trabecular (85%) and uveoscleral (5-15%) pathways, and, partially, through the iris) as well as through the posterior pathways (through the vitreous body in the perineural spaces of the optic nerve and perivascular spaces of the retinal vascular system) [23, 13]. Since the aqueous humor is produced by the ciliary processes in the posterior chamber and, afterwards, flows to the anterior chamber [3, 1], the excess of peroxidation products can get into the aqueous humor from ciliary body, iris, and other tissues due to various pathogenic factors (ocular hypertension, inflammation, etc.). Thus, our findings reveal an important link in the pathogenic action of elevated IOP which burdens inflammation in the uveal tissues through the activation of oxidative and peroxidative processes. In addition, our findings support the assumption that primary high-pressure glaucoma can be a factor worsening inflammation in the anterior eye. References 1.Erichev VP, Tumanov VP, Panyushkina LA. [Glaucoma and neurodegenerative disease]. Glaukoma. 2012;1:62–68. In Russian. 2.Zavgorodniaia NG, Pasechnikova NV. [Primary glaucoma: A new look at an old problem]. Zaporizhzhia: Orbita-YUG; 2010. 192 p. In Russian. 3.Nesterov AP. [Glaucoma]. M.: MIA; 2008. 360p. In Russian. 4.Pasyechnikova NV, Rykov SA, Naumenko LIu, Kryzhanovskaia TV. [Prevention of Blindness and Visual Impairment in Ukraine (implementation of the WHO’s Vision 2002 program)]. In: [Proceedings of the Conference on Current Issues of Ophthalmology]. 2009, Dnipropetrovsk: 8-11. In Russian. 5.Flammer J, Orgul S, Costa VP. The impact of ocular blood flow in glaucoma. Ibid. 2002; 21: 359–393. 6.Likhvantseva VG, Gabibov AA, Solomatina MV, Belogurov AA, Korosteleva EV, Vygodin VA. [The role of immune reactions in the pathogenesis of optic neuropathy in normal tension glaucoma]. Natsionalnyi Zhurnal Glaukoma. 2014;13(2):17-28. In Russian. 7.Sokolov VA, Mkhinini N, Levanova ON. [Autoimmune mechanisms in the pathogenesis of the primary open angle glaucoma]. Rossiiskii meditsinsko-biologicheskii vestnik. 2011;2:154-9. In Russian. 8.Beishenova GA, Chesnokova NB. [A role of free radical oxidation in the pathogenesis of uveitis]. Rossiiskii oftalmologicheskii zhurnal. 2015;9(2):99-105. In Russian. 9.Gazizova IR. [The state of the oxidation-reduction system in patients with primary open angle glaucoma]. Kazanskii meditsinskii zhurnal. 2012;3:488-90. In Russian. 10.Kurysheva NI. [Contribution of free-radical reactions of chamber humor to the development of primary open-angle glaucoma]. Vestn Oftalmol. 1996 Sep-Oct;112(4):3-5. In Russian. 11. Mikheitseva IN. [Lipid peroxidation in experimental adrenaline-induced glaucoma]. Oftalmol Zh. 1989;(7):427-8. In Russian. 12. Dinh QN, Drummond GR, Sobey ChG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed. Res. Int. – 2014; 2014:406960. 13.Boland MV, Quigley HA. Risk factors and open-angle glaucoma: classifications and application. J. Glaucoma. 2007;16(4):406-18. 14.Ko M, Peng P, Ma M. Dinamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic. Biol. Med. 2005;39:365 – 3. 15.Yelskiy VN, Mikheytseva IN. [Disregulatory aspects of glaucoma process (review of literature and own data)]. Zhurnal NAMN Ukrainy. 2011;17(3):235-44. In Russian. 16.Mikheytseva IN, Yelskiy VN. [Stress-induced dysregulation in glaucoma process and protective infl uence of melatonin]. Patologiia. 2011;8(2):66-8. In Russian. 17.Wang YY. Experimental study of carbomer glaucoma model in rabbits by injecting different location in anterior chamber. Ophthalmol. 2009;45: 1 - 95. 18.Urvantseva GA, Gracheva EL. [Methods of analysis of living systems: guidance]. Iaroslavl:IarGU; 2013:104p. In Russian. 19.Fried R, Fried L. Xanthin-Oxydase (Xanthin-Dehydrogenase). In: H. U. Bergmeyer. Methoden der enzymatischen analyse. Berlin: Аcademie Verlag, 1984. B. I.:625 – 629. 20.Orekhovych VN. [Current approaches in biochemistry]. Moscow: Meditsyna; 1977. 392p. In Russian. 21.Nasledov A. [SPSS computer analysis of data in psychology and social studies]. St.-Petersburg: Piter Publ. 2005:416. In Russian. 22.Rebrova ОY. [Statistical analysis of medical data. Usage of the package of application programs STATISTICA]. M.: Media Sphera. 2002:312. In Russian. 23.Bettin P, Matteo FDi. Glaucoma: present challenges and future trends. Ophthalmic Res. 2013;50:197–208.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|