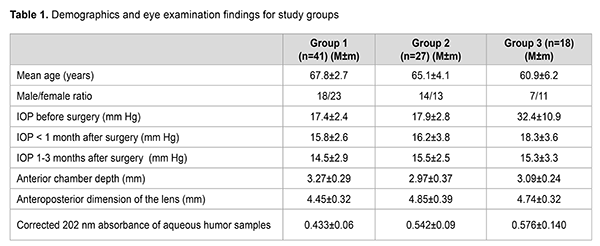J.ophthalmol.(Ukraine).2019;2:3-6.
|
http://doi.org/10.31288/oftalmolzh2019236 Received: 05 April 2018; Published-online: 23 April 2019 Spectroscopic analysis of intraocular fluid in patients with cataract and pseudoexfoliation syndrome-associated glaucoma P.A. Bezditko,1 Dr Sc (Med), Prof.; V.O. Melnyk,2 Cand Sc (Med); S.V. Kolotilov,3 Dr Sc (Chem) 1 Kharkiv National Medical University; Kharkiv (Ukraine) 2 Visiobud+ Clinic LLC; Kyiv (Ukraine) 3 Pisarghevskiy Institute of Physical Chemistry of NAS of Ukraine; Kyiv (Ukraine) E-mail: volo_mel@ukr.net, visiobud@ukr.net TO CITE THIS ARTICLE: Bezditko PA, Melnyk VO, Kolotilov SV. Spectroscopic analysis of intraocular fluid in patients with cataract and pseudoexfoliation syndrome-associated glaucoma. J.ophthalmol.(Ukraine).2019;2:3-6. http://doi.org/10.31288/oftalmolzh2019236 Background: Pseudoexfoliation syndrome (PES) is a risk factor for the development of primary open angle glaucoma (POAG). Impaired intraocular fluid (as reflected by the chemical composition and physical properties, velocity and viscosity, of this fluid) is a major factor for elevated intraocular pressure (IOP). Purpose: To assess anterior eye characteristics and intraocular fluid protein levels in patients with PES and to compare them with those in patients with glaucoma and patients with cataract only. Materials and Methods: We assessed anterior eye characteristics (anterior chamber depth and crystalline lens size) and protein levels in the intraocular fluid obtained intraoperatively during lens exchange in patients with PES. These data were compared with those of patients with glaucoma and patients with cataract only. Results: The changes in anterior eye characteristics and intraocular fluid protein levels in patients with cataract plus PES were similar to those in patients with glaucoma, but not to those in patients with cataract without PES. Conclusion: Study findings advocate for early surgical cataract extraction to avoid the development of glaucoma in patients with PES. Keywords: open angle glaucoma, pseudoexfoliation syndrome, anterior chamber depth, crystalline lens size, spectroscopy of the intraocular fluid Introduction Glaucoma is the major cause of irreversible blindness worldwide. The number of people with glaucoma worldwide will increase to 111.8 million in 2040. The leading cause of reversible blindness is cataract accounting for 33% of visual disability worldwide. A major risk factor for both these diseases is age, and their comorbidity is expected to be common among older patients [1]. Glaucoma is an optic neuropathy and major cause of blindness affecting about 60 million people worldwide. The greatest risk factor for development of primary open angle glaucoma (POAG) is elevated intraocular pressure (IOP). The primary goal of glaucoma treatment is to reduce IOP either by pharmacological, laser or surgical means with an aim of preventing or reducing the rate of progressive optic neuropathy [2]. Phacoemulsification has been found to reduce IOP in eyes with open angle of the anterior chamber. In the study by Poley et al [1, 3,] the final mean IOP reduction was 6.5 mm Hg in the 31 to 23 mm Hg presurgical IOP Group and 4.8 mm Hg in the 22 to 20 mm Hg Group. The mechanism of IOP reduction after phacoemulsification remains unclear. Poley et al hypothesize that age-related thickening of the crystalline lens narrows the anterior chamber angle and contributes to the onset of pressure elevation and eventually glaucoma. They propose that pressure elevation associated with lens expansion should be considered as a type of phacomorphic ocular hypertension [4]. Issa et al found that pressure lowering after phacoemulsification was highly correlated with preoperative IOP and the anterior chamber depth. However, they did not find a statistically significant relationship between IOP reduction and lens thickness [5]. Aqueous humor contains 0.5% to 1% of proteins, and is formed from plasma by epithelial cells of the ciliary body. The main aqueous humor characteristic most affected by the protein content is the viscosity [6]. Changes in aqueous humor protein composition (e.g., due to pseudoexfoliation) may affect aqueous humor hydrodynamics, since elevation of proteins leads to an increase in the viscosity and reduction in the flow of aqueous humor and, consequently, an increase in the pressure it exerts on the walls of the eye. Glycoproteins and proteoglycans are among the most common proteins in aqueous humor of pseudoexfoliation eyes [6, 7]. Beer–Lambert–Bouguer’s law states that the absorbance of a non-ideal fluid is directly proportional to concentration of the absorbing species. The 202 nm absorbance of aqueous humor samples from eyes with both cataract and glaucoma has been found to be higher than that from eyes with cataract only. This was evidence for elevated protein concentration in glaucomatous eyes, thus leading to increased viscosity and reduced flow of fluid, and to increased IOP [8]. These findings imply that there might be elevated protein content in the anterior chamber of pseudoexfoliation eyes, which might affect the development of glaucoma in these eyes. The purpose of the study was to use spectroscopy for determining the aqueous humor proteins levels in eyes of patients with pseudoexfoliation syndrome (PES), and to compare them with those from patients with glaucoma and patients with cataract without signs of PES. Materials and Methods Of the 86 patients (99 eyes) included in the study, 47 (55%) were women (mean age, 66.9 years), and 39 (45%) were men (mean age, 65.2 years). Patients with moderate or severe myopia or moderate or severe hyperopia were excluded from the study. Informed consent was obtained as per the Helsinki Protocol. Patients were divided into three groups. Group 1 involved cataract-only patients (41 eyes) without visible signs of PES who had conventional phacoemulsification and intraocular lens (IOL) implantation. Group 2 (27 eyes) involved patients with cataract plus PES, and without signs of glaucoma, who underwent conventional phacoemulsification and IOL implantation. Group 3 (18 eyes) involved patients with cataract plus POAG plus PES, who had modified tunnel trabeculopuncture (MTTP) with phacoemulsification and IOL implantation [9]. All procedures were performed by a single surgeon between 0.4.04.2017 and 15.06.2017. Methodology of the study All patients underwent an examination within a month before surgery. Patients with POAG received ocular hypotensive eye drops preoperatively; however, they differed in types and regimens of these drops. In addition, each patient had instillation of an antibacterial agent one or two days before and for seven days after surgery and instillation of topical anesthetic, proparacaine hydrochloride 0.5% (Alcaine®, Alcon Laboratories, Fort Worth, Tex.), before surgery and intraoperatively. A sample of aqueous humor (50 to 200 μL) was collected using a syringe with a 26-G needle intraoperatively, immediately after anterior chamber paracentesis, transferred to a sterile Eppendorf tube, and transported to the laboratory. Before AH sampling, the ocular surface was rinsed with saline (0.9% NaCl) and dried with surgical sponges. Postoperatively, there were no signs of infectious complications potentially related to intraoperative contamination. Samples were neither frozen nor sterilized before being kept in the fridge at 5°C, and were analyzed within 24 hours of collection. We scanned the AH samples in a Specord 210 Analytic (Jena AG, Germany) spectrophotometer to produce graphs of the sample absorbance versus radiation wavelength in nanometers (nm), over the wavelength range 190-1.100 nm. Patients underwent an examination both before and within 3 months after surgery. Anterior chamber characteristics and lens size were assessed preoperatively. In addition, patients underwent Goldman applanation tonometry before and after surgery. Microsoft Excel 2011 for Mac and SPSS software were used for statistical analyses. The level of significance p ≤ 0.05 was assumed. Results The demographics and eye examination findings of study patients are reported in Table 1. Preoperatively, the IOPs of patients in Group 1 and Group 2 were substantially lower than that of patients in Group 3. Patients of Group 3 had MTTP with phacoemulsification and IOL implantation since they had high IOP (32.4±10.9 mm Hg) and optical coherence tomographic and perimetric evidence of POAG [9]. There were no intraoperative or postoperative complications. Patients in Group 1 and Group 2 exhibited an insubstantial decrease in IOP (by 20% and 15%, respectively) at month 1 compared to baseline, which is consistent with findings of others on a decreased IOP after phacoemulsification [5]. Patients in Group 3 exhibited a substantial decrease in IOP (by more than 50%, to 15.3±3.3 mm Hg) due to the surgical procedure, MTTP with phacoemulsification and IOL implantation.
There were no significant differences (р > 0.05) between Group 2 and Group 3 with respect to crystalline lens size, anterior chamber depth and corrected AH absorbance (2.97 mm vs 3.09 mm; 4.85 vs 4.74 mm; and 0.542 vs 0.576, respectively). However, there were significant differences (р < 0.05) in these characteristics between Group 1 (3.27 mm; 4.45 mm; and 0.433, respectively), on the one hand, and groups 2 and 3, on the other hand. These findings indicate similarity in anterior eye characteristics and aqueous humor protein composition between patients with PES and those with POAG. The obtained results suggest a high risk for developing POAG in patients with PES, since an increased concentration of protein molecules in aqueous humor evidences an increased aqueous humor viscosity and, correspondingly, a decreased aqueous humor flow resulting in elevated IOP. Changes in the anatomical characteristics of the anterior eye and aqueous humor protein composition in early pseudoexfoliation syndrome might not result in an immediate increase in IOP due to decreased aqueous humor production. However, a prolonged reduction in aqueous humor flow and increase in its viscosity due to increased number of colloid protein molecules results in alterations in aqueous humor outflow, including those at the level of trabecular meshwork and Schlemm's canal, which leads to elevated IOP and glaucoma. It is interesting that the protein molecules refracting light at 202 nm were found to be represented in aqueous humors of patients of all groups, that is, this protein is not specific just for patients with PES. However, the concentration of this protein for Groups 2 and 3 was statistically significantly higher than that for Group 1. This might be evidence for a decreased flow of fluid, which is the major hydrodynamic factor for elevated IOP. This conclusion results from Bernoulli's flaw for non-ideal fluids, according to which, the fluid viscosity increase is directly proportional to the pressure the fluid exerts on the walls of the vessels it travels through. Therefore, anterior eye changes and aqueous humor composition changes typical of patients with POAG are also found in pseudoexfoliation syndrome patients without well-marked signs of glaucoma. Based on these data, it may be believed that anatomical changes in anterior eye are a driver of elevated IOP, which result in the emergence of barriers to fluid flow, leading to a local of generalized reduction in pressure gradient in the system. A reduction in pressure gradient causes a reduced fluid flow, and, correspondingly, an increased fluid viscosity and increased pressure the fluid exerts on the walls of the eye. The pseudoexfoliations accumulated in anterior eye structures are a sign of a reduced fluid flow and impaired fluid hydrodynamics. We noted a relationship between the presence of pseudoexfoliations, on the one hand, and the changes in lens characteristics and anterior chamber depth, on the other hand. Patients with PES were noted to have a relatively shallow anterior chamber and increased anteroposterior dimension of the crystalline lens. In the absence of well-marked signs of glaucoma, normalization of anterior eye characteristics through phacoemulsification may be a prophylactic measure against the development of glaucoma associated with PES. A reduction in IOP after phacoemulsification in patients with PES was also encouraging. Conclusion A spectroscopic study found evidence of an increased number of protein molecules per volume unit in the aqueous humor of patients with PES, with no difference from that in the aqueous humor of patients with POAG. These findings suggest that, in patients with PES, phacoemulsification may be a prophylactic measure against the development of POAG due to changes in the anatomical characteristics of the anterior eye, which improves aqueous humor flow, reduces IOP, and, correspondingly, reduces the risk of the development of POAG. References 1. Armstrong JJ, Wasiuta T, Kiatos E, et al. The effect of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: A systematic review and meta-analysis of 3-year data. J Glaucoma. 2017 Jun;26(6):511-522. 2. Al-Mugheiry TS, Cate H, Clark A, et al. Microinvasive glaucoma stent surgery (MIGS) with concomitant phakoemulsification cataract extraction: Outcomes and the learning curve. J Glaucoma. 2017 Jul;26(7):646-651. 3. Poley BJ, Lindstrom RL, Samuelson TW. Long-term effect of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg. 2008 May;34(5):735-42. 4. Zetterström C, Behndig A, Kugelberg M, et al. Changes in intraocular pressure after cataract surgery: analysis of the Swedish National cataract register data. J Cataract Refract Surgery. 2015 Aug;41(8):1725-9. 5. Issa de Fendi L, Cena de Oliveira T, Bigheti Pereira C, et al. Additive effect of risk factors for trabeculectomy failure in glaucoma patients. J Glaucoma. 2016 Oct;25(10):e879-e883. 6. Gabelt BT, Kaufman PL. Aqueous humor hydrodynamics. In: Hart WM, editor. Adler’s Physiology of the eye. 9th ed. St Louis: MO: Mosby; 2003. 7. Koliakos GG, Konstas AGP, Holló G. Biochemistry and genetics of exfoliation syndrome. In: Holló G, Konstas AGP, editors. Exfoliation Syndrome and Exfoliative Glaucoma. 3rd ed. Savona: PubliComm; 2012. 8. Kolotilov SV, Melnyk VO, Lytvynenko AS, et al. Comparison of Spectroscopic Properties of Intraocular Fluid in Patients with Cataract and Primary Open-Angle Glaucoma. Fiziol Zh. 2016;62(5):62-8. 9. Mel’nik VO, Kots-Gotlib NV, Vladiuk RL, Hurzhii OO. Assessment of the efficacy of the combined surgical technique for primary open-angle glaucoma and age-related cataract. J Ophthalmol (Ukraine).2016;3:28-30.
The authors certify that they have no conflicts of interest in the subject matter or materials discussed in this manuscript.
|