J.ophthalmol.(Ukraine).2019;1:46-51.
|
http://doi.org/10.31288/oftalmolzh201914651 Received: 17 November 2018; Published on-line: 28 February 2018 Neuro-ophthalmological symptoms in pituitary apoplexy K.S. Iegorova, Cand Sc (Med); M.O. Guk, Dr Sc (Med); O.Ie. Skobska, Dr Sc (Med); L.V. Zadoianyi, Cand Sc (Med) Romodanov Neurosurgery Institute; Kyiv (Ukraine) E-mail: iegorova_katya@ukr.net
Background: Neuro-ophthalmological symptoms in patients with pituitary apoplexy were subjected to analysis. Impaired circulation in pituitary adenoma causes an atypical clinical course of the disease which makes the early diagnosis and provision of medical care difficult. Neuro-ophthalmological manifestations of the disease include loss of vision, visual field defects and oculomotor disturbances. Since the visual loss may present as unilateral or bilateral blindness, the field of this disease is of special importance. Purpose: To investigate neuro-ophthalmological symptoms in patients with pituitary apoplexy before and after treatment. Materials and Methods: Ninety patients with pituitary apoplexy were under surveillance at the Romodanov Neurosurgery Institute between 2014 and 2017. Of these, 44 patients (88 eyes) had visual loss and/or visual field defects, and were included in the main group of the study. Patients underwent clinical and neurological, neuro-ophthalmological, and otoneurological examination (including neuroimaging studies). Results: Acute pituitary apoplexy (54.5% of patients) was characterized by sudden onset of presumably symmetric (50% of patients) chiasmal syndrome, and manifested by mildly or moderately impaired BCVA (39.6% of eyes) and sensitivity to light (60% of eyes), partial bitemporal hemianopia, oculomotor disturbances and absence of ophthalmoscopic changes. In addition, surgical treatment resulted in restoration of visual acuity and visual field in 27 (56.3%) eyes. Subacute pituitary apoplexy (45.5% of patients) was characterized by a gradual development of chiasmal syndrome, with a history of sudden visual function loss, and by presumably severe or very severe visual acuity loss (65% of eyes), light sensitivity loss (67.5% of eyes), and development of primary descending optic nerve atrophy (70% of eyes). In addition, surgical treatment resulted in improvements in visual function in most (65%) eyes; however, compared with acute pituitary apoplexy, a complete restoration of visual function was less frequently observed (15%). Conclusion: Pituitary apoplexy is one of the causes of acute visual loss in neurosurgical disorders. In acute pituitary apoplexy, early diagnosis and surgical treatment resulted in stabilization or improvement in visual acuity from 0.54±0.05 to 0.81±0.03 (p < 0.05), and improvement in visual field defects (as assessed by MD values) from 9.05±0.99 to 4.18±0.81 dB (p < 0.05). Keywords: pituitary adenoma, pituitary apoplexy, chiasmal syndrome, optic nerve atrophy
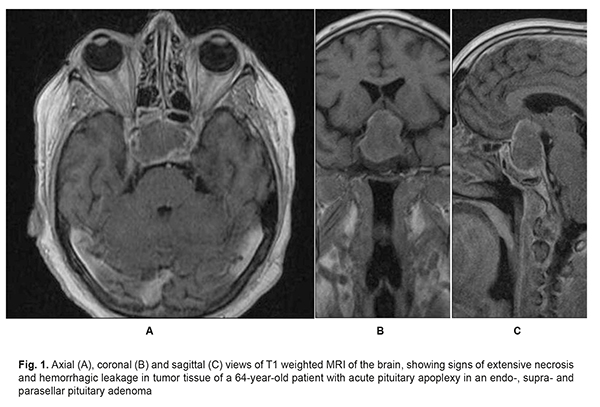
Introduction Pituitary apoplexy is a clinical condition characterized by sudden headache, nausea, vomiting, impaired consciousness, visual and oculomotor disturbances, and meningeal symptoms resulting from impaired circulation in pituitary adenoma. Changes in tumor tissue such as necrosis, hemorrhagic leakage, hemorrhage, formation of hematomas and intratumoral hemorrhagic cysts result in a rapid increase in tumor volume and changes in tumor relationships with adjacent brain structures [1- 3] (Fig. 1).
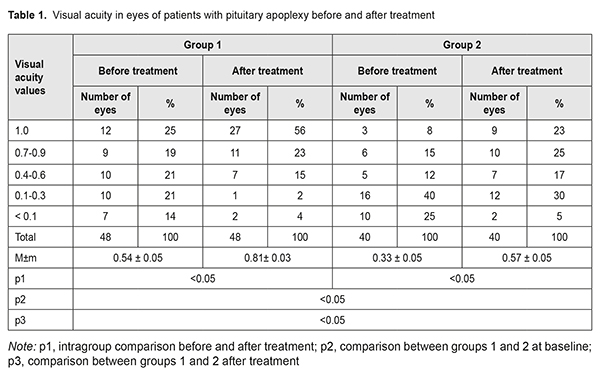
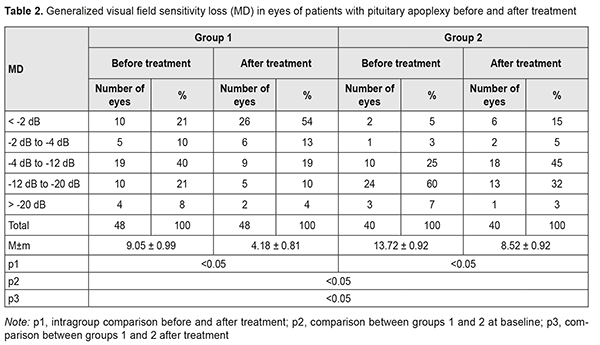
Pituitary adenomas account for 12-15% of all intracranial neoplasms, and pituitary apoplexy is a rare emergency that occurs in a small but significant percentage (0.6-16.6%) of patients with pituitary adenomas [4-9]. Typically, the clinical course of pituitary adenoma is characterized by a gradual development of chiasmal syndrome that represents the major portion of the clinical picture of the disease. Chiasmal compression by the neoplasm results in a slow reduction in visual acuity, characteristic bitemporal visual field defects and descending primary atrophy of the optic nerve [8]. Impaired circulation in pituitary adenoma (pituitary apoplexy) causes an atypical clinical course of the disease which includes general brain symptoms, sudden substantial loss of visual function, and oculomotor disturbances. This determines a high (above 45%) rate of false primary diagnosis of stroke or meningoencephalitis, which substantially worsens outcomes. Severe visual function impairment and persistent oculomotor symptoms are more common in delayed surgical interventions [8, 10]. The most common symptom of pituitary apoplexy is headache, with the incidence of 90-100%. Neuro-ophthalmic manifestations are seen in 78-82.6% of cases of pituitary apoplexy, and include loss of vision, visual field defects and oculomotor disturbances [1, 9, 11, 12]. Since the visual loss may present as unilateral or bilateral blindness in 35-45% of patients with pituitary apoplexy, the field of this disease is of special importance [4, 13, 14]. The purpose of the study was to investigate neuro-ophthalmological symptoms in patients with pituitary apoplexy before and after treatment. Materials and Methods The records of 90 patients (41 women (45.6%) and 49 men (54.6%); age, 20 to 77 years; mean age, 54 ± 1.9 years) who received treatment for pituitary apoplexy at the Romodanov Institute during 2014 to 2017 were used in this retrospective study. Of these, 44 patients (88 eyes) had visual loss and/or visual field defects, and were included in the main group of the study. The inclusion criterion was surgical evidence of pituitary adenoma with signs of pituitary apoplexy. Patients underwent clinical and neurological, ophthalmological, and otoneurological examination (a routine otoneurological examination with assessment of cranial nerve function). Instrumental and laboratory studies were conducted. Neuroimaging included sella turcica X-ray study with AXIOM Iconos R100 (Siemens) or Radrex-I (Toshiba) in 72 patients, magnetic resonance imaging (MRI) of the brain with a 1.5-T MRI system (Intera 1.5T/I system, Philips Medical Systems, Best, the Netherlands) or 0.2-T MRI system (Magnetom Concerto, Siemens Medical Solutions, Erlangen, Germany) in 14 patients and computed tomography (CT). The MRI of brain and pituitary gland were obtained using T1-weighted image (WI) and T2WI. In 90 cases (81% of patients), a T1-weighted brain MRI scan was acquired post intravenous administration of 0.2 mL/kg gadolinium. Neuro-ophthalmilogical examination included visual acuity assessment, biomicroscopy, kinetic and static perimetry, and direct and indirect ophthalmoscopy. Best-corrected visual acuity was classified as normal (1.0), mild impairment (0.7-0.9), moderate impairment (0.4-0.6), severe impairment (0.1-0.3), and very severe impairment (< 0.1). Static automated perimetry was performed with the Centerfield 2 Perimeter (Oculus, Wetzlar, Germany) using the neurological 30-2 threshold test program and Neuro screening program. Aside from defect localization, the arithmetic mean of the sensitivity loss, the mean defect (MD), was used to assess visual field loss severity. Visual field loss severity was classified as “no visual field loss” (Grade 0; normal visual field), mild visual field loss (Grade 1; MD, –2 dB to –4 dB), moderate visual field loss (Grade 2; MD, –4 dB to –12 dB), severe visual field loss (Grade 3; MD, –12 dB to –20 dB), and very severe visual field loss (Grade 4; MD, worse than –20 dB). The visual field loss was classified as very severe if it was not possible to assess visual fields due to the extremely poor visual function. A chiasmal syndrome was considered symmetric if both eyes had the same grade of visual field loss. In addition, a chiasmal syndrome was considered asymmetric if the difference between eyes in grade of visual field loss severity was 1, and it was considered markedly asymmetric if the difference was 2 or greater. Eye movement limitation in four directions of gaze was scored as no limitation = 0 points; ?1-mm limitation = 1 point; 1/3 limitation = 2 points; 1/2 limitation = 3 points; 2/3 limitation = 4 points; and no eye movement = 5 points. Visual acuity was assessed with a diaphragm if there was mydriasis caused by oculomotor nerve palsy. The data were statistically processed using Statistica 6.0 (StatSoft, Tulsa, OK). Results are presented as the mean and standard deviation (M ± SD). Student’s unpaired t test was used to determine differences between independent groups. The level of significance p ? 0.05 was assumed. Results and Discussion A total of 894 patients with pituitary adenoma underwent surgery during 2014 по 2017. Of these, 90 (9.9%) had pituitary apoplexy, and were included for the analysis. Fifty eight (64.4%) had visual loss, visual field loss, and/or oculomotor disturbances, which evidenced the presence of various directions of tumor extension, with compression of the optic nerve/chiasm complex by the suprasellar portion of the mass and lesions in the intracavernous portions of cranial nerves III, IV and VI. Visual loss and/or visual field defects were found in 44 patients (48.8%); of these, 15 had also oculomotor impairments. Oculomotor nerve pareses, but not visual loss and/or visual field defects were found in 29 patients (32.2%). Otoneurological examination revealed impaired function of the nervus ophthalmicus in 13 patients (14.5%). Patients with visual function loss (n=44) were divided into two tentative groups, group 1 (acute pituitary apoplexy) and group 2 (subacute pituitary apoplexy). Stress and increased blood pressure were promoting factors in most (60%) patients of both groups. Pituitary adenoma was classified as suprasellar (27 patients; 61.4%), supra- and parasellar (10 patients; 22.7%), supra- and infrasellar (4 patients; 9.1%), and supra-, infra-, and parasellar (3 patients; 6.8%) with regard to tumor extension. In addition, it was classified as hormonally inactive in 36 (81.8%) patients, and prolactin-, growth hormone- and adrenocorticotropic hormone-secreting, in 6 (13.6%), 1 (2.3%), and 1 (2.3%), respectively. Group 1 (those with acute disease onset) included 24 (54.5%) patients (48 eyes). They had sudden headache, loss of consciousness, loss of vision, and diplopia. In this group, all patients had decreased vision in one or both eyes; in 12 (50%) patients, only one eye had a VA lower than 1.0; in 7 (29.2%), both eyes had a VA lower than 1.0; in 3 (12.5%), one eye had a VA lower than 1.0, and another, a VA lower than 0.1; in 2 (8.3%), both eyes had a VA lower than 0.1. Best-corrected visual acuity (BCVA) was normal (1.0), mildly impaired (0.7-0.9), moderately impaired (0.4-0.6), severely impaired (0.1-0.3), and very severely impaired (< 0.1) in 12 (25%), 9 (18.8%), 10 (20.8%), 10 (20.8%) and 7 (14.6%) eyes, respectively. In addition, two patients had a blind eye. Static perimetry found no changes in 10 (20.8%) eyes. Temporal hemianopia (either complete or partial) only was the commonest field defect (15 eyes; 31.3%), followed by temporal hemianopia with central scotoma (10 eyes; 20.8%), central scotoma only (7 eyes; 14.6%), residual visual field in the nasal inner quadrant, with a complete loss of central vision (2 eyes; 4.2%), and nasal hemianopia (1 eye; 2%). Visual field was not measurable due to extremely low visual function in 3 (6.3%) eyes. Loss of sensitivity to light was classified as mild in 5 (10.4%) eyes, moderate in 19 (49.6%) eyes, and severe and very severe in 10 (20.8%) eyes and 4 (8.3%) eyes, respectively. No loss of sensitivity to light was seen in 10 (20.8%) eyes. Symmetric, asymmetric or markedly asymmetric chiasmal syndrome was found in 12 (50%), 9 (37.5%), and 3 (12.5%) patients, respectively. Ophthalmoscopy found retinal angiopathy in 17 patients (34 eyes), but no eye demonstrated optic nerve atrophy. Oculomotor disturbances were observed in 14 patients, including 6 with abducens nerve palsy, 5 with oculomotor nerve palsy, and 3 with oculomotor and abducens nerve palsies. Group 2 (those with subacute disease onset) included 20 (45.5%) patients (40 eyes). They complained most commonly of gradually decreased vision with a history of acute headache accompanied by progressive visual disturbance. In group 2, all patients had decreased vision in one or both eyes; in 4 (20%) patients, only one eye had a VA lower than 1.0; in 9 (45%), both eyes had a VA lower than 1.0; in 5 (25%), one eye had a VA lower than 1.0, and another, a VA lower than 0.1; in 2 (10%), both eyes had a VA lower than 0.1. Best-corrected visual acuity was normal (1.0), mildly impaired (0.7-0.9), moderately impaired (0.4-0.6), severely impaired (0.1-0.3), and very severely impaired (< 0.1) in 3 (7.5%), 6 (15%), 5 (12.5%), 16 (40%) and 10 (25%) eyes, respectively. In addition, two patients had a blind eye. Symmetric, asymmetric or markedly asymmetric chiasmal syndrome was found in 11 (55%), 5 (25%), and 4 (20%) patients, respectively. Static automated perimetry found no visual field changes in 2 (5%) eyes. Temporal hemianopia (either complete or partial) only was the commonest field defect (19 eyes; 47.5%), followed by temporal hemianopia with central scotoma (12 eyes; 30%), and residual visual field in the nasal inner quadrant, with a complete loss of central vision (5 eyes; 12.5%). Visual field was not measurable in 2 (5%) eyes. Loss of sensitivity to light was classified as mild in 1 (2.5%) eye, moderate in 10 (25%) eyes, and severe and very severe in 24 (60%) eyes and 3 (7.5%) eyes, respectively. No loss of sensitivity to light was seen in 2 (5%) eyes. Ophthalmoscopy found primary descending optic nerve atrophy, retinal angiopathy, and no changes in 14 (70%) patients (27 eyes), 5 (25%) patients (10 eyes), and 1 (5%) patient (2 eyes), respectively. Oculomotor disturbances were observed in one patient with oculomotor nerve palsy. All patients underwent transsphenoidal surgery at various time points after the onset of disease due to delayed patient arrival to the tertiary care unit. In group 1, BCVA maintained at 1.0 in 12 (25%) eyes, restored to 1.0 in 15 (31.3%) eyes, improved in 18 (37.5%) eyes, did not change in 3 (6.2%) eyes, and worsened in no eyes after surgery (Table 1), and mean BCVA improved from 0.54±0.05 at baseline to 0.81±0.03 after treatment (the improvement was significant, p < 0.05). In addition, visual field remained normal in 10 (20.8%) eyes, restored to normal in 17 (35.4%) eyes, improved in 19 (49.6%) eyes, did not change in 2 (4.2%) eyes, and worsened in no eyes. Moreover, mean overall visual field sensitivity loss changed significantly (p < 0.05) after treatment (Table 2). After surgery, 5 patients demonstrated complete resolution of oculomotor deficits, and 9 patients with incomplete resolution of oculomotor deficits underwent a restorative treatment. In group 2, BCVA maintained at 1.0 in 3 (7.5%) eyes, restored to 1.0 in 6 (15%) eyes, improved in 26 (65%) eyes, did not change in 4 (10%) eyes, and worsened in 1 (2.5%) eye after surgery (Table 1), and mean BCVA improved from 0.33±0.05 at baseline to 0.57±0.05 after treatment (the improvement was significant, p < 0.05). In addition, visual field remained normal in 2 (5%) eyes, restored to normal in 5 (12.5%) eyes, improved in 27 (67.5%) eyes, did not change in 5 (12.5%) eyes, and worsened in 1 (2.5%) eye. Moreover, MD changed significantly (p < 0.05) after treatment (Table 2), and all patients demonstrated resolution of oculomotor deficits.
Therefore, we found a variety of visual deficits and their combinations with oculomotor symptoms in a large cohort of patients with pituitary apoplexy. Acute pituitary apoplexy (54.5% of patients) was characterized by sudden onset of presumably symmetric (50% of patients) chiasmal syndrome, and manifested by mildly or moderately impaired BCVA (39.6% of eyes) and sensitivity to light (60% of eyes), partial bitemporal hemianopia, oculomotor disturbances and absence of ophthalmoscopic changes. Surgical treatment resulted in restoration of visual function in 27 (56.3%) eyes, with improvements in visual acuity and visual field in 18 (37.5%) and 19 (49.6%) eyes, respectively. Subacute pituitary apoplexy (45.5% of patients) was characterized by a gradual development of chiasmal syndrome, with a history of sudden visual function loss, and by presumably severe or very severe visual acuity loss (65% of eyes), light sensitivity loss (67.5% of eyes), and development of primary descending optic nerve atrophy (70% of eyes). In addition, surgical treatment resulted in improvements in visual function in most (65%) eyes; however, compared with acute pituitary apoplexy, a complete restoration of visual function was less frequently observed (15%). Persistent compression of the optic nerve/chiasm complex by the tumor that rapidly and substantially increases in size in pituitary apoplexy, and poor chiasmal blood supply due to impaired circulation in pituitary adenoma may result in irreversible ischemic and atrophic changes in the optic nerve. Conclusion Pituitary apoplexy syndrome is one of the causes of acute visual loss in neurosurgical disorders. It was found that in impairment in the circulation in pituitary adenoma, changes in the circulation may be either ishemic or hemorrhagic. In addition, visual function becomes impaired due to not only the onset of or increase in compression of the optic nerve/chiasm complex, but also poor chiasmal blood supply caused by vasospasm-related and/or steal syndrome-related impairment in chiasmal circulation. In acute pituitary apoplexy, early diagnosis and surgical treatment resulted in stabilization or improvement in visual acuity from 0.54±0.05 to 0.81±0.03 (p < 0.05), and improvement in visual field defects (as assessed by MD values) from 9.05±0.99 to 4.18±0.81 dB (p < 0.05). References
1.Simon S, Torpy D, Brophy B, Blumbergs P, Selva D, Crompton J.L. Neuro-ophthalmic manifestations and outcomes of pituitary apoplexy – a life and sight-threatening emergency. N Z Med J. 2011 May 27;124(1335):52-9. 2.Biousse V, Newman NJ, Oyesiku NM. Precipitating factors in pituitary apoplexy. J Neurol Neurosurg Psychiatry. 2001 Oct;71(4):542-5. 3.Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, Wass J.A. Classical pituitary apoplexy: clinical features, management and outcome. Clin Endocrinol (Oxf). 1999 Aug;51(2):181-8. 4.Turgut M, Ozsunar Y, Basak S, Guney E, Kir E, Meteoglu I. Pituitary apoplexy: an overview of 186 cases published during the last century. Acta Neurochir (Wien). 2010 May;152(5):749-61. 5.Wakai S, Fukushima T, Teramoto A, Sano K. Pituitary apoplexy: its incidence and clinical significance. J Neurosurg. 1981 Aug;55(2):187-93. 6.Dubuisson AS, Beckers A, Stevenaert A. Classical pituitary tumour apoplexy: clinical features, management and outcomes in a series of 24 patients. Clin Neurol Neurosurg. 2007 Jan;109(1):63-70. 7.Bonicki W, Kasperlik-Za?uska A, Koszewski W, Zgliczy?ski W, Wis?awski J. Pituitary apoplexy: endocrine, surgical and oncological emergency. Incidence, clinical course and treatment with reference to 799 cases of pituitary adenomas. Acta Neurochir (Wien). 1993;120(3-4):118-22. 8.Guk MO. [Diagnosis and treatment of non-functioning pituitary adenomas]. [Dr Sc (Med) dissertation]. Kyiv: Romodanov Neurosurgery Institute; 2017. 328 p. Ukrainian. 9.Serova NK. [Clinical neuroophthalmology: Neurosurgical aspects]. Tver’: Triada Publishing House; 2011. Russian. 10.Chuang CC, Chang CN, Wei KC, Liao CC, Hsu PW, Huang YC, Chen YL, Lai LJ, Pai PC. Surgical treatment for severe visual compromised patients after pituitary apoplexy. J Neurooncol. 2006 Oct;80(1):39-47. 11.Bi WL, Dunn IF, Laws ER Jr. Pituitary apoplexy. Endocrine. 2015 Feb;48(1):69-75. 12.Ayuk J, McGregor EJ, Mitchell RD, Gittoes NJ. Acute management of pituitary apoplexy – surgery or conservative management? Clin Endocrinol (Oxf). 2004 Dec;61(6):747-52. 13.Agrawal D, Mahapatra AK. Visual outcome of blind eyes in pituitary apoplexy after transsphenoidal surgery: A series of 14 eyes. Surg Neurol. 2005 Jan;63(1):42-6. 14.Muthukumar N, Rossette D, Soundaram M, Senthilbabu S, Badrinarayanan T. Blindness following pituitary apoplexy: timing of surgery and neuro-ophthalmic outcome. J Clin Neurosci. 2008 Aug;15(8):873-9.
|