J.ophthalmol.(Ukraine).2019;1:33-38.
|
http://doi.org/10.31288/oftalmolzh201913338 Received: 17 December 2018; Published on-line: 28 February 2019 Intraoperative changes in intraocular temperature during vitrectomy procedures with irrigating solutions differing in temperature L.I. Anatychuk,1,2 Member of the NAS of Ukraine, Dr Sc (Phys and Math), Prof.; N.V. Pasyechnikova,3 Assoc Member of the NAMS of Ukraine, Dr Sc (Med), Prof.; V.A. Naumenko,3 Dr Sc (Med), Prof.; R.E. Nazaretian,3 ophthalmologist; M.M. Umanets,3 Dr Sc (Med); R.R. Kobylianskyi,1,2 Cand Sc (Phys and Math); O.S. Zadorozhnyy,3 Cand Sc (Med) 1 Institute of Thermoelectricity of the National Academy of Science and Ministry of Education and Science, Ukraine; Chernivtsi (Ukraine); 2 Yuriy Fedkovych Chernivtsi National University; Chernivtsi (Ukraine); 3 Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine; Odessa (Ukraine); E-mail: laserfilatova@gmail.com TO CITE THIS ARTICLE: Anatychuk LI, Pasyechnikova NV, Naumenko VA, Nazaretian RE, Umanets MM, Kobylianskyi RR, Zadorozhnyy OS. Intraoperative changes in intraocular temperature during vitrectomy procedures with irrigating solutions differing in temperature. J.ophthalmol.(Ukraine).2019;1:33-8.http://doi.org/10.31288/oftalmolzh201913338 Background: Intraoperative intraocular media and irrigation fluid temperature monitoring is not performed during vitreoretinal surgery in current clinical practice. Purpose: To investigate intraoperative changes in intraocular temperature at the major steps of vitreoretinal surgery with irrigating solutions differing in temperature. Materials and Methods: Thirty-nine patients (39 eyes) were under observation before, during and after vitrectomy. Group 1 (20 patients; 20 eyes) and Group 2 (19 patients; 19 eyes) differed with respect to the baseline temperature (24.2 ± 0.52 °С, or 10.3 ± 1.1 °С, respectively) of the irrigating fluid. Room air temperature, irrigating fluid temperature, patient’s body temperature, blood pressure, heart rate and blood oxygen saturation, vitreous temperature, and duration of every step of the vitreoretinal procedure were recorded in all cases. Results: Vitrectomy with 24°C or 10°C irrigating solutions resulted in a substantial decrease in temperature in vitreous compartments to the level of moderate or deep hypothermia, respectively. In addition, immediately after vitrectomy, the lowest temperatures were found in the anterior vitreous, and were 30.1 ± 0.45 °С for the 24°C group and 24.37±0.52 °С for the 10°C group. Moreover, after additional surgical manipulations, in the presence of discontinued irrigation, the temperatures in the vitreous compartments were found to gradually increase. Conclusion: Vitreoretinal surgery is performed under conditions of induced uncontrolled local hypothermia, which requires intraoperative monitoring of intraocular temperature and irrigating solution temperature. During a vitroretinal procedure lasting up to 30 minutes, the intraocular contents may be safely cooled to the level of deep hypothermia. Keywords: vitroretinal surgery, intraocular temperature, human eye
Introduction Intraoperative intraocular media and irrigation fluid temperature monitoring is not performed during vitreoretinal surgery in current clinical practice. In addition, it remains poorly understood what should be the temperature of the irrigating solution for intraocular surgery and how long it is reasonable to use irrigating solutions during vitrectomy [1]. The temperature of irrigating solutions used during surgical procedures commonly conforms to the ambient temperature in the operating room [2, 3]. Therefore, vitreoretinal surgery is performed under conditions of induced local hypothermia. A number of authors believe it reasonable to use low-temperature irrigating fluids in vitreoretinal surgery. Thus, as early as 1986, Rinkoff et al [4] demonstrated in rabbits that a fluid of a temperature below that of the body can be used in vitreoretinal surgery to reduce retinal damage from endoilluminator light. In the study by Tamai and colleagues [5], after the rabbits underwent closed vitrectomy, their vitreous cavities were irrigated with a low-temperature solution at a perfusion pressure of 140 mm Hg to induce ischemia in the rabbit eye. In addition, the least changes in the retinal structural and electrophysiological characteristics compared to controls were observed in the low-temperature group. Compared to control eyes, experimental eyes demonstrated less intraocular bleeding volume, less fibrin production, and less postoperative inflammation in the Jabbour et al [6] study evaluating local ocular hypothermia with irrigating balanced salt solution cooled to 7 °С in rabbits. Romano and colleagues [7] found that the variations in temperature during vitreoretinal surgery are clinically significant, as the rheology of tamponades can be better manipulated by modulating intraocular pressure and temperature. In our previous rabbit study [8], in the 22°C or 5°C irrigation solution groups, after vitrectomy with 60-minute continuous vitreous cavity cooling, there have been light microscopy evidence of retinal structural changes in the form of uneven edema in the inner and outer retinal layers, and these changes were more common and more substantive in the latter group. However, after vitrectomy with 30-minute continuous vitreous cavity cooling, there has been no evidence of retinal structural changes in the rabbit eyes [1]. Therefore, by monitoring the intraocular temperature and the duration of exposure time of low-temperature irrigating solution the intraocular structures are exposed to during vitreoretinal surgery conducted under hypothermic conditions, thermal changes in the eye may be able to be controlled. This in turn will result both in improved use of beneficial effects of local hypothermia and reduced rates of intra- and post-operative complications in eye disease management. The purpose of the study was to investigate intraoperative changes in intraocular temperature at the main steps of vitreoretinal surgery with irrigating solutions differing in temperature. Materials and Methods This was an open pilot study. The study protocol was approved by a local Bioethics Committee of the Filatov Institute. Written informed consent was obtained from all individual participants included in the study. Thirty-nine patients (39 eyes, including 16 with diabetic retinopathy, 4 with vitreous hemorrhage, 15 with rhegmatogenous retinal detachment, and 4 with macular tear; age, 37 to 65 years) were under observation before, during and after vitrectomy. They were divided into two groups, Group 1 (20 patients; 20 eyes) and Group 2 (19 patients; 19 eyes), which differed with respect to the baseline temperature of BSS PLUS® (Alcon Laboratories, Inc., Fort Worth, USA; either room temperature of 24.2±0.52 °С, or 10.3±1.1 °С, respectively) used for irrigation. 23-G pars plana vitrectomy was performed using the Alcon Constellation vitrectomy machines. Technique The surgical site was prepared with antiseptic solution and epibulbar and subtenon anesthesia was administered. Thereafter, a standard three-port vitrectomy was performed with cutting rates of 3500-7000 cuts/min, aspiration pressure of 300-650 mm Hg, and irrigation pressure of 25 mm Hg. The temperature of the irrigating solution delivered into the eye was monitored and controlled during surgery. Cold (10 °С) fluid was prepared by cooling the solution inside the irrigating tube with gel packs that were located outside the tube, and thus cooling was performed in close proximity to the surgical site. Room-temperature (24 °C) fluid was obtained by placing the bottles with solution in the operating room for several hours before surgery. The mean operating room temperature before surgery was 24.4 ± 0.51 °С. Room air temperature, and patient’s body temperature, blood pressure, heart rate and blood oxygen saturation were recorded in all cases. Temperatures in the anterior, mid- and posterior vitreous were measured before vitrectomy, immediately after surgery, and after each additional manipulation such as endolaser retinal photocoagulation, removal of the internal limiting membrane (ILM), and retinal flattening with perfluorodecalin. The timing of every step of vitreoretinal surgery was recorded for each patient. A thermoelectric device [9, 10] developed by the Institute of Thermoelectricity of the NAS of Ukraine and MES of Ukraine, and the Filatov Institute was used for measuring temperatures of various ocular structures, irrigating solution, and operating room. Patients were supervised for 5-7 days immediately after surgery, and had follow-up visits at 1 and 3 months. Statistics Means and standard deviations (SD) were calculated. The level of significance p ≤ 0.05 was assumed. For between group comparisons, the Student's t-test was used for normally distributed and the Mann-Whitney test was used for non-normal data. For pairwise comparison of temperatures in different vitreous compartments, repeated measures ANOVA with Bonferroni correction were used for normally distributed data, whereas Friedman test for related samples and Conover tests were used for non-normal data. Logistic regression models were used to examine the relationships of baseline vitreous temperature with patient’s diagnosis, body temperature, heart rate and blood oxygen saturation. Regression analysis was used to identify the relationship of a change (Δ) in midvitreous temperature at the time point after additional surgical manipulations, compared to the time point immediately after vitrectomy), with time for additional surgical manipulations. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) and MedCalc 18.10 (MedCalc Software Inc, Broekstraat, Belgium) software.
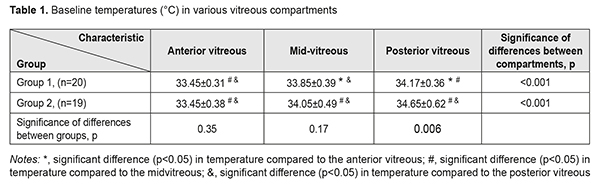
Results At the first stage of the study, vitreous temperatures were measured in both groups at baseline (before vitrectomy). There was a significant transvitreous temperature gradient (p < 0.001, rANOVA) from the lens toward the retina (Table 1). Posterior vitreous temperatures were higher than mid- and anterior vitreous temperatures. There was no association of baseline vitreous temperature with patient’s diagnosis, body temperature, blood pressure, heart rate or blood oxygen saturation (p > 0.05). Mean patient body temperatures in groups 1 and 2 were 36.58±0.08 °С and 36.6±0.08 °С, respectively (p =0.44).
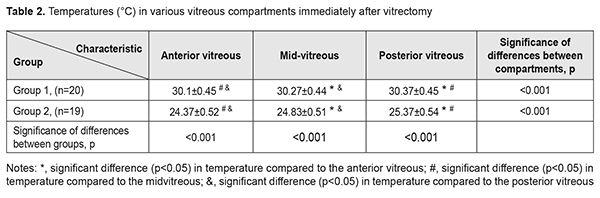
At the second stage of the study, vitreous temperatures were measured in both groups immediately after vitrectomy. Mean durations of vitrectomy in groups 1 and 2 were 6.4±0.75 min and 6.7±1.1 min, respectively (p =0.3). Immediately after vitrectomy (time point 2), in groups 1 and 2, posterior, mid- and anterior vitreous temperatures were significantly lower compared to baseline (p < 0.001, Table 2), and posterior vitreous temperatures were higher than mid- and anterior vitreous temperatures. In addition, temperatures in each compartment of the vitreous in group 2 were substantially (by approximately 5 °С) lower than those in group 1 (p<0,001). Table 2 presents the results of the analysis of mean temperature measurements for the three vitreous compartments in both groups at time point 2.
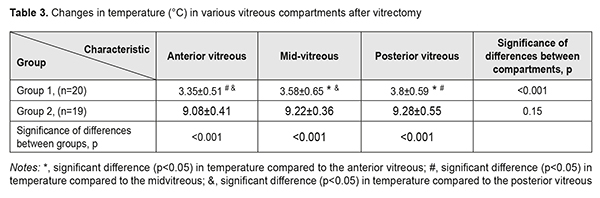
Table 3 presents changes in temperature for the three vitreous compartments in both groups immediately after vitrectomy compared to baseline. In group 1, the lowest temperature decrease (3.35 ± 0.51 °С) was for the anterior vitreous, and the highest (3.8 ± 0.59 °С), for the posterior vitreous (p<0.05). We found no statistically significant differences in temperature decrease between various vitreous compartments (p=0.15) among patients of group 2. In addition, among patients of group 2, a magnitude of temperature decrease (8.4 °C – 10.5 °C) after vitrectomy for each vitreous compartment was more substantial than that among patients of group 1 (2.4 °C – 5.9 °C; p<0.001).
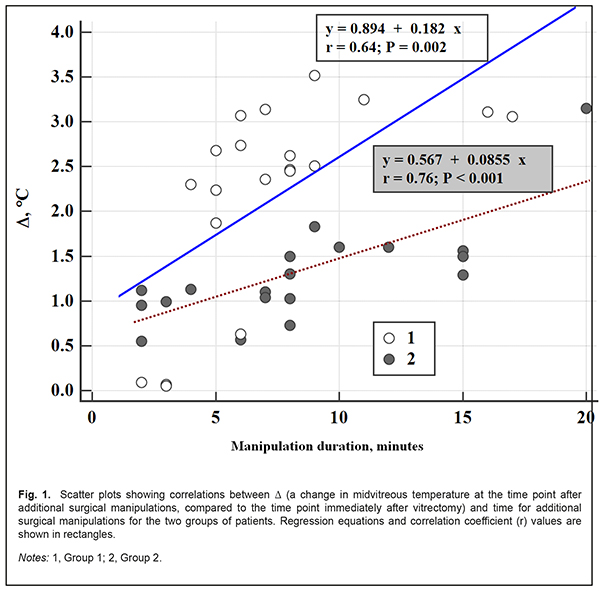
After additional surgical manipulations, the increase in midvitreous temperature compared to immediately after vitrectomy in group 1 was greater than in group 2 (2.21±1.11 °С vs 1.29±0.57 °С, p=0.004) and there was no significant between-group difference in terms of duration of additional surgical manipulations (7.3±3.9 min vs 8.15 ± 5.1 min, p=0.46). There was a positive linear relationship between change in midvitreous temperature after additional surgical manipulations and duration of additional surgical manipulations both for group 1 (r=0.64, p=0.002) and group 2 (r=0.76, p < 0.001). Therefore, in both groups, vitreous temperatures increased with an increase in duration of manipulations, but they increased faster among patients of group 1 than among patients of group 2 (at an average rate of 0.18 °С per minute vs 0.085 °С per minute). In addition, in both groups, during and after vitreoretinal surgery, there were no complications (like retinal detachment or tear, vitreous hemorrhage, or endophthalmitis) that could be attributable to the introduction of additional probes into the vitreous. Moreover, no corneal changes were observed, and lens clarity was maintained in all eyes during surgery. Discussion Therapeutic controlled hypothermia has been successfully applied in various medical fields (like cardiac surgery, neurosurgery, and resuscitation science) for improving brain cell resistance to ischemic conditions [11-15]. The neuroprotective efficacy of therapeutic hypothermia is directly related to the decrease in temperature and the decrease in oxygen consumption by neurons [16]. Hypothermia can be classified based on the depth of cooling from a normal body temperature of 37–38°C. Mild hypothermia describes a body temperature in the range 32–35°C, moderate hypothermia (28–32°C), and deep hypothermia (<28°C) [13]. Mild threrapeutic hypothermia in critical care patients has been found to be effective for neuroprotection, with improvements in overall survival and neurologic outcomes [14, 17]. Moderate hypothermia has been widely used in cardiac surgery for brain protection. It has been reported [18] that deep hypothermia (24–26°C) allows for a safe prolong (>60 minutes) cardiac arrest in patients with severe heart disease or aortic arch disease. Total body cooling may result in side effects as severe as marked cardiovascular system depression [7, 12]. These side effects limit the safe level of body cooling, and, correspondingly, the degree of neuroprotective effect provided by therapeutic hypothermia. In the current study, we found that application of room temperature irrigating solutions during intraocular surgery resulted in a substantial decrease in vitreous temperature to the level of moderate local hypothermia. In addition, application of 10 °С irrigating solutions resulted in a decrease in intraocular temperature to the level of deep (below 28 °С) local hypothermia. Although the magnitude of between-group difference in the temperature of irrigating fluid was approximately 14 °С, the magnitude of between-group difference in vitreous temperature after vitrectomy with continuous vitreous cavity irrigation was just 5 °С (with no between-group differences in vitrectomy duration and baseline intraocular temperatures). This may indicate that choroidal blood flow is an important contributor to the maintenance of intraocular heat balance. In addition, the groups were found to differ in the increase in vitreous temperature after discontinuance of irrigation. After the irrigating fluid was turned off, vitreous temperature recovered to baseline levels in group 1 at a rate (0.18 °С /min) greater than in group 2 (0.085 °С /min), probably also due to the capacity of choroidal blood flow for maintenance of heat balance in the eye. Moreover, there were no complications during or after vitroretinal procedures lasting up to 30 minutes, which confirmed our previous experimental findings [1]. Therefore, during a vitroretinal procedure, the intraocular contents may be safely cooled to the level of deep hypothermia, which might confer additional neuronal protection and homeostasis. Nevertheless, further research is warranted to determine optimal conditions (target temperature, duration, and re-warming rate) for the induction of intraoperative controlled local ocular hypothermia during vitrectomy. Conclusion First, vitreoretinal surgery is performed under conditions of induced uncontrolled local hypothermia, as the irrigation fluid temperature is lower than that of the intraocular media, and intraoperative intraocular temperature monitoring is not common practice. Second, vitrectomy with application of irrigating solutions of 24°C or 10°C resulted in a substantial decrease in temperature in vitreous compartments to the level of moderate or deep hypothermia, respectively. In addition, immediately after vitrectomy, the lowest temperatures were found in the anterior vitreous, and were 30.1±0.45 °С for the 24°C group and 24.37±0.52 °С for the 10°C group. Third, the rate of an increase in vitreous temperature after completion of vitrectomy and discontinuance of irrigation was found to depend on the temperature of irrigating fluid. Thus, vitreous temperatures increased faster among patients undergoing vitrectomy with the 24°C irrigating solution than with the 10°C irrigating solution (at an average rate of 0.18 °С per minute vs 0.085 °С per minute). Finally, during a vitroretinal procedure lasting up to 30 minutes, the temperature of the irrigating fluid may be safely lowered to 10°С. References
|