J.ophthalmol.(Ukraine).2019;1:56-60.
|
http://doi.org/10.31288/oftalmolzh201915660 Received: 19 December 2018; Published on-line: 28 February 2019 OCT angiography for diagnosing and postoperative monitoring of serous maculopathy associated with optic disc pit N.S. Lutsenko,1 Dr Sc (Med), Prof.; O.A. Rudycheva,1 Cand Sc (Med); O.A. Isakova,1 Cand Sc (Med); A.N. Sergienko,2-3 Dr Sc (Med), Prof 1 Zaporizhzhia Medical Academy of Post-Graduate Education; Zaporizhzhia (Ukraine) 2 Professor Sergienko Eye Clinic; Vinnytsia (Ukraine) 3 ВNational Medical University; Vinnytsia (Ukraine) E-mail: rudychevaolga5@gmail.com TO CITE THIS ARTICLE: Lutsenko NS, Rudycheva OA, Isakova OA, Sergienko AN. OCT angiography for diagnosing and postoperative monitoring of serous maculopathy associated with optic disc pit. J.ophthalmol.(Ukraine).2019;1:56-60.http://doi.org/10.31288/oftalmolzh201915660
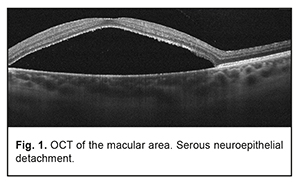
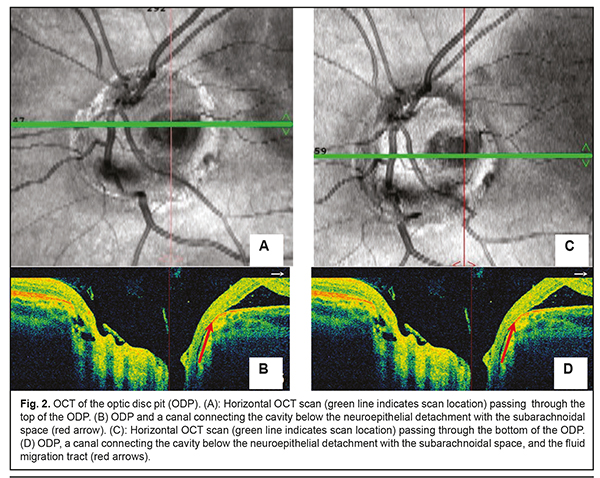
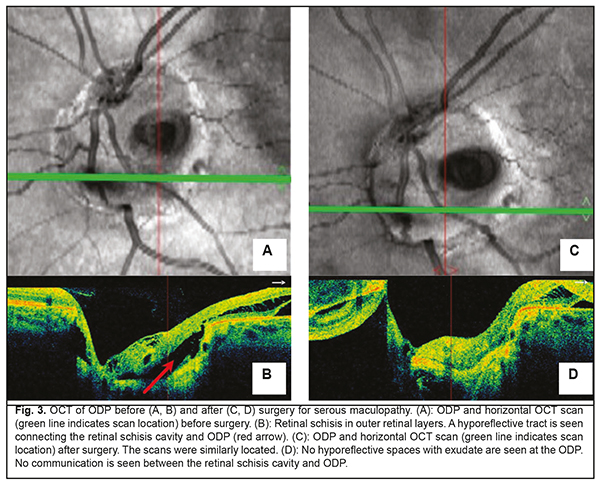
Background: Optic disc pits (ODP) usually present with the serous maculopathy found in 30% to 75% of these patients. Purpose: To demonstrate the potential of optical coherence tomography angiography (OCTA) for monitoring the natural and postoperative course of ODP maculopathy. Materials and Methods: We present a case of a 27-year-old female patient with serous maculopathy associated with optic disc pit. OCTA scans were obtained using RTVue XR Avanti (Optovue, Fremont, CA). The OCTA-based vessel density was assessed. The follow-up after presentation was 10 months. Results: The development of maculopathy in this case was found to be associated with penetration of liquor from the subarachnoidal space through the intermembranous spaces of the optic nerve. It is important to monitor the pathological areas found with the use of OCT and OCTA not only intraoperatively, but also postoperatively. Complete blockage of fluid tracts was achieved, evidencing the efficacy of treatment measures. A decrease in vessel densities of the superficial retinal plexus in the foveal and parafoveal regions of the affected eye was accompanied by deterioration of visual functions, and guided the choice of treatment strategy. OCTA enables monitoring of morphological changes before and after surgical treatment and assist in making a prognosis for visual function. Keywords: optic disc pit, serous maculopathy, optical coherence tomography angiography Introduction Optic disc pit (ODP) maculopathy is a consequence of communication between the intermembranous spaces and subretinal cavity due to congenital dyspasia and nerve tissue deficit. ODPs are rare, and occur with an estimated incidence of 1 in 11,000 people. Maculopathy occurs in 30–75% of patients with an ODP and develops commonly in individuals aged approximately 20 to 40 years [1-3]. Most of the data on ODP are available as case reports or case series with the size of a case series ranging from two to twenty cases [3-6]. Optical coherence tomography (OCT) is the main objective non-invasive method used to monitor retinal changes including those related to the diagnosis of ODP and its complications. Although fluorescein angiography and indocyanine green angiography are capable of revealing the detachment of the macular neuroepithelium, which is seen as a delineated site of hypofluorescence [7] during the late phase, they provide not enough information to reveal changes in serous retinal detachment. The invasiveness of the two methods limits their wide use for long-term monitoring of retinal changes. Histologically, an ODP is a herniation of elements of neurosensory retina at the defect in the lamina cribrosa. Retinal fibers go inside the pit, and then come back and go outside in front of the incoming optic nerve. In some cases, ODP has a communication with the subarachnoidal space. The development of serous neuroepithelial detachment is attributed to either the passage of intraocular fluid beneath the retina at the ODP or penetration of liquor from the subarachnoidal space through the intermembranous spaces of the optic nerve. Some researchers believe that elevated intracranial pressure triggers the development of retinal detachment associated with ODP [1, 3]. There is no general consensus on the best treatment for ODP and its complications. The European VitreoRetinal Society (EVRS) Optic Pit study evaluated clinical characteristics and outcome of treatments for optic pit maculopathy, and showed no significant benefits of any treatment as compared to other treatments. The treatments proposed aim at creating an effective barrier to prevent fluid migration from the ODP to the macula (or from the intermembranous spaces to the intraretinal spaces), or removing fluid leakage through the retina. Conservative treatment involving dehydration therapy and topical corticosteroids is symptomatic and of low efficacy. Lee and Peyman [8] were the first to propose vitrectomy as a treatment for ODP maculopathy. The efficacy of internal limiting membrane (ILM) flap techniques and combinations of various tamponading agents is being investigated to improve surgical approaches to management of ODP maculopathy [1, 3, 9-13]. Recurrent maculopathy and long-term resorption of subretinal fluid are the issues to be solved while applying any method for treating ODP maculopathy. The purpose was to demonstrate the potential of optical coherence tomography angiography (OCTA) for monitoring the natural and postoperative course of ODP maculopathy. Clinical case A 27-year-old woman presented with a 2-week history of gradually decreasing vision in the left eye. At the time of presentation, she was undergoing a course of conservative therapy (involving dehydration agents, osmotic diuretics, corticosteroids, and non-steroidal anti-inflammatory drugs) with no improvement in vision. On examination, uncorrected visual acuity (UCVA) was 1.0 in the right eye and 0.3 in the left eye, and the best-corrected visual acuity (BCVA) in the left eye was 0.4. The peripheral visual field was normal in each eye. There was a central left eye scotoma of 5 to 10 degrees in size (with a total area of 50 degrees). The IOP in each eye was 18 mm Hg. Anterior segment was unremarkable and the media were transparent OU. Lenses were used for indirect ophthalmoscopy with the slit lamp. Ophthalmoscopy of the right eye showed no pathological changes. Ophthalmoscopy of the left eye showed an enlarged pale pink optic disc with grey round excavation on the temporal side. The macula was flat. Macular edema and no macular reflex were seen. The patient’s left eye was imaged on OCT and OCTA with the RTVue XR Avanti (Optovue, Fremont, CA) using 3D, en-face and widefield images. OCTA was performed in 3 mm x 3 mm and 6 mm x 6 mm macular regions for the optic disc of 4.5 mm, and analyses were performed on OCTA images. Vessel density was assessed and expressed in percentage by taking the ratio of the total vessel area to the total area of analyzed region. Measurements were performed in foveal and parafoveal sectors. The right eye showed no pathological changes. Macular OCT revealed a large serous neuroepithelial detachment (Fig. 1) with intraretinal schisis cavities (Fig. 3b). The OCT of the optic disc and peripapillary region revealed that the detachment approached the temporal optic disc margin where an ODP was identified. A canal was seen connecting the cavity below the neuroepithelial detachment with the subarachnoidal space (Fig. 2). Retinal schisis was found in the external retinal layers and extended from the temporal optic disc margin to the nasal aspect of the fovea. A canal was observed connecting the retinal schisis cavity with the optic disc (Fig. 3 a, b). The resulting image was partially inverted (Figs. 2b, 2d and 3d) since a large axial range had to be represented at the OCT scan. ODP with serous maculopathy in the left eye was finally diagnosed based on examination findings.
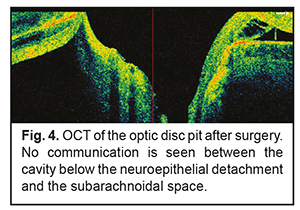
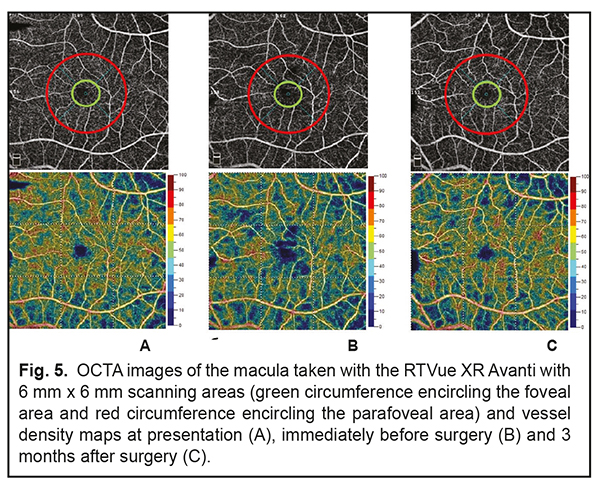
Findings of additional studies and examinations were as follows: A blood panel was normal. A magnetic resonance imaging of the brain, orbits and chiasm was normal. The patient was evaluated by a neuropathologist who detected no neurological disorder. The neuroepithelial detachment tended to increase in height and area over time during the course of a five-month monitoring of the pathological process. At baseline, there was OCTA evidence of a noteworthy difference in vessel density of the superficial retinal plexus in the foveal region between the affected eye (39.46%) and the healthy eye (44.64%), but the difference in vessel density in the parafoveal region was rather small (57.62% vs 56.67%, respectively). During the first four months, vessel densities of the superficial retinal plexus in the foveal and parafoveal regions of the affected eye were practically stable (37.09% and 57.03%, respectively), whereas visual functions did not change from baseline values. However, during the fifth month, vessel densities of the superficial retinal plexus in the foveal and parafoveal regions of the affected eye decreased to 24.59 % and 48.24%, respectively. In addition, the visual density in the affected eye decreased to 0.1, which made the patient to agree for a surgical intervention. Therefore, a surgical intervention was conducted in the presence of a progressive pathological process and decreasing visual function 5 months after making a diagnosis. The patient underwent a 23-G three-port vitrectomy with the temporal inverted internal limiting membrane (ILM) flap technique of Nawrocki. The procedure was initiated with a standard 3-port pars plana vitrectomy, including removal of the posterior hyaloid. Thereafter, membranes were stained with Membrane Blue Dual. A wide temporal ILM flap was fashioned and used to cover the ODP. Air-and-gas tamponade was performed to make the flap attached to the pit. The patient was advised to maintain face-down position for three days postoperative. At 1 month following vitrectomy, the UCVA in the left eye improved to 0.4 (the BCVA was 0.6). In addition, OCT-measured neuroepithelial detachment height decreased, and OCT showed the ILM flap. Moreover, OCT found no evidence of the hyporeflective tract connecting the retinal schisis cavity with the optic disc (Fig. 3 c. d) or ODP communication with the subarachnoidal space (Fig. 4). There was OCTA evidence of increased vessel density of the superficial retinal plexus in the foveal region (41.8%) and in the parafoveal region (55.72%).
The patient was followed up for 5 months postoperatively. Discussion Serous neuroepithelial detachments occur commonly in the presence of a temporal optic disc pit, and extend temporally from the optic disc, but not beyond the vascular arcades, which is consistent with our case [1, 3]. The clinical picture of serous neuroepithelial detachment is similar to that of central serous chorioretinopathy, making diagnosing a challenge. Therefore, the distinction between central serous chorioretinopathy and serous neuroepithelial detachment requires an optic disc study or widefield OCT imaging. The differential diagnosis should also include diffuse epitheliopathy and retinal schisis of other etiology. The patient did not seek medical attention before because patients with this congenital condition maintain high visual acuity for long before a macular problem arises. Establishment of the pathogenesis of the serous maculopathy and exclusion of potential contributors to complications and recurrence will assist in making a prognosis for visual function and disease course and choosing between alternative treatments. The development of serous neuroepithelial detachment is attributed to either the passage of intraocular fluid beneath the retina at the ODP or penetration of liquor from the subarachnoidal space through the intermembranous spaces of the optic nerve. That is, serous neuroepithelial detachment may originate in the vitreous cavity, blood vessels at the bottom of optic disc pit, or the subarachnoidal space. There have been reports on OCT studies evaluating ODP, cavities, lamina cribrosa defects, and potential origins of serous maculopathy [1, 14]. In the current case, we used OCTA to follow changes in the retinal capillary bed. To the best of our knowledge, this is the first report to evaluate retinal vessel density using OCTA in a patient with this disorder. In the current case, there was no OCT or OCTA evidence of the communication between the retinal schisis area and the vitreous cavity. In addition, OCT and OCTA found no blood vessels at the bottom of optic disc pit, and no retinal neovasculatization. However, the affected patient’s eye was found to have a canal for passage of intraocular fluid from the suprachoroidal space to beneath the retinal neuroepithelium (see arrows in Fig. 2). In addition, it was found to have a canal for passage of intraocular fluid from the subarachnoidal space to an area of retinal schisis. We believe that the pathological areas found with the use of OCT and OCTA should be monitored not only intraoperatively, but also postoperatively. An intraretinal barrier preventing fluid migration completely, if created, would be evidence for the efficacy of treatment measures, and would contribute to further improvement of the disease. However, if the ophthalmologist fails to achieve complete blockage of fluid tracts, a repeat intervention will be required. OCT of the retina at 3 months after surgery (Fig. 4) demonstrated disappearance of (a) communication with the submembranous space which was present at baseline, and (b) the cavity containing transudate at the ODP which was communicating with retinal schisis cavities at baseline (Fig. 3c). The residual fluid found below the neuroepithelial detachment is gradually reabsorbed, and this process may continue for a year or longer. OCTA found changes in vessel density of the superficial retinal plexus in the foveal region over the follow-up period. As the pathological process progressed and the height of neuroepithelial detachment increased, the vessel density of the superficial retinal plexus in the foveal region decreased, whereas as the process regressed, the density restored (Fig. 5) and remained practically stable until the end of the follow-up. However, follow up should be prolonged, as these pathological changes in vessel density of the superficial retinal plexus in the foveal region may lead to degenerative retinal changes [15, 16].
Further research is warranted to investigate this rare disease and to optimize OCTA procedure though the use of most suitable retinal scanning modes and algorithms for interpretation of detected pathology. Conclusion If serous maculopathy is identified, OCT of optic disc area must be performed for the differential diagnosis of the optic disc pit. OCT allows for diagnosing and enables monitoring of changes in retinal tissues in optic disc pit, and visualizing the potential canals of fluid migration between various macular layers. Postoperative OCT enables assessing the resolution of optic disc tissue defects and thus the efficacy of surgical treatment. OCTA is an important non-invasive adjunct for the diagnosis, monitoring the course of the disease, and assessment of the efficacy of surgical treatment. OCTA-based assessment of retinal circulation seems promising for clinical use for evaluating changes in the pathological process in the presence of therapy. References
1.Bayborodov YV, Izmailov AS. [The detachment of the retina caused by the pit of the optic nerve disk and its surgical treatment]. Fyodorov Journal of Ophthalmic Surgery. 2017;(4):20-5. Russian 2.Ganichenko IN. [Photo and laser coagulation for management of optic disc pit and its complications]. Oftalmol Zh. 1986;4:199. Russian. 3.Moisseiev E, Moisseiev J, Loewenstein A. Optic disc pit maculopathy: when and how to treat? A review of the pathogenesis and treatment options. Int J Retina Vitreous. 2015 Aug 7;1:13. Available 4. Apple DJ, Rabb MF, Walsh PM. Congenital anomalies of the optic disc. Surv Ophthalmol. 1982 Jul-Aug;27(1):3-41. 5.Brown GC, Shields JA, Goldberg RE. Congenital pits of the optic nerve head II. Clinical studies in humans. Ophthalmology. 1980 Jan;87(1):51–65. 6.Gass JDM. Serous detachment of the macula secondary to congenital pit of the optic nerve head. Am J Ophthalmol.1969;67:821–49. 7.Theodossiadis GP, Ladas ID, Panagiotidis DN, Kollia AC, Voudouri AN, Theodossiadis PG. Fluorescein and indocyanine green angiographic findings in congenital optic disk pit associated with macular detachment. 1999;19(1):6-11. 8.Lee KJ, Peyman GA. Surgical management of retinal detachment associated with optic nerve pit. Int Ophthalmol. 1993 Apr;17(2):105-7. 9.Georgalas I, Papaconstantinou D, Koutsandrea C, et al. Optic disc pit maculopathy: the value of small-gauge vitrectomy, peeling, laser treatment, and gas tamponade. Eur J Ophthalmol. 2013;23:275. 10.Ghosh YK, Banerjee S, Konstantinidis A, Athanasiadis I, Kirkby GR, Tyagi AK. Surgical management of optic disc pit associated maculopathy. Eur J Ophthalmol. 2008 Jan-Feb;18:142-6. 11.Hirakata A, Inoue M, Hiraoka T, McCuen BW 2nd. Vitrectomy without laser treatment or gas tamponade for macular detachment associated with an optic disc pit. Ophthalmology. 2012 Apr;119(4):810-8. 12.Rizzo S, Belting C, Genovesi-Ebert F, et al. Optic disc pit maculopathy: the value of small-gauge vitrectomy, peeling, laser treatment, and gas tamponade. Eur J Ophthalmol. 2012;22:620–5. 13.Sandali O, Barale PO, Bui Quoc E, et al. [Long-term results of the treatment of optic disc pit associated with serous macular detachment: a review of 20 cases]. J Fr Ophtalmol. 2011 Oct;34(8):532-8. 14.Ohno-Matsui K, Hirakata A, Inoue M, Akiba M, Ishibashi T. Evaluation of congenital optic disc pit and optic disc colobomas by Swept-Source optical coherence tomography. Invest Ophthalmol Vis Sci. 2013 Nov 25;54(12):7769-78. 16.Schatz H, McDonald HR. Treatment of sensory retinal detachment associated with optic nerve pit or coloboma. 1988 Feb;95(2):178-86. 17.Lumbroso B, Huang D, Chen CJ, et al., editors. Clinical OCT Angiography Atlas. New Delhi: Jaypee Brothers Medical Publishers; 2015.
|