J.ophthalmol.(Ukraine).2019;1:3-8.
|
http://doi.org/10.31288/oftalmolzh2019138 Received: 14 November 2018; Published on-line: 27 February 2019
Relationship of the recurrence after pterygium surgery with the presence of HSV, EBV, CMV, HPV, and BRAFV600E mutation S.O. Rykov1, Dr Sc (Med), Prof.; K.O Usenko1, MD; S.Iu. Mogilevskyy1, Dr Sc (Med), Prof.; S.V. Ziablitsev2, Dr Sc (Med), Prof.; L.I. Denisiuk1, Cand Sc (Med) 1 Shupik National Medical Academy of Postgraduate Education, Kyiv; Kyiv (Ukraine) 2 Bogomolets National Medical University; Kyiv (Ukraine) E-mail: sergey.mogilevskyy@gmail.com TO CITE THIS ARTICLE: RykovSO, UsenkoKO, MogilevskyySIu, ZiablitsevSV, DenisiukLI. Relationship of the recurrence after pterygium surgery with the presence of HSV, EBV, CMV, HPV, and BRAFV600E mutation. J.ophthalmol.(Ukraine).2019;1:3-8. http://doi.org/10.31288/oftalmolzh2019138 Background: Previously, we have reported on associations of pterygium with the presence of herpes simplex virus (HSV), Epstein Barr virus (EBV), cytomegalovirus (CMV), human papillomavirus (HPV), and the BRAFV600E somatic point. The current study attempted to examine the relationship between the recurrence during 1 year after pterygium surgery and the presence of these factors. Purpose: To identify the relationship between (a) recurrence rates after various surgical techniques for pterygium and (b) the presence of herpes viruses (HSV, EBV, and CMV), HPV 6, 11, 16 and 18 and BRAFV600E mutation. Materials and Methods: Two hundred and three patients (232 eyes) with a history of pterygium surgery underwent examination. They were divided into five groups based on the method of surgical treatment: Group 1 (the McReynolds transplantation only) of 49 eyes; Group 2 (the McReynolds transplantation, with adjuvant MMC 0.02% applied to the cornea for 30 sec) of 41 eyes; Group 3 (the technique of Arlt) of 49 eyes; Group 4 (the technique of Arlt, with adjuvant MMC 0.02% applied to the cornea for 30 sec) of 46 eyes; Group 5 of 47 eyes that were treated with our technique of pterygium excision involving the growth plate, with conjunctival autoplasty and adjuvant MMC 0.02%. Real-time PCR with PCR kits from DNK Tekhnologiya (Russia) were used to identify HSV, EBV, CMV, and HPV. PCR amplification was performed in a DT-lite real-time PCR system (DNK Tekhnologiya). Mutation detection was performed using TaqMan Mutation Detection Assays (Thermo Fisher Scientific) in a Real-Time PCR System 7500 (Applied Biosystems, USA). Results: Changing from traditional surgical methods (the McReynolds or Arlt method, alone or with adjunctive mitomycin C) to our methodology for pterygium decreased the recurrence rate at 1 year after pterygium surgery by 17.0% (or 2.1-fold), from 31.9% to 14.9% (χ2 = 5.32; р = 0.021). There was a common trend regarding the relationship between the presence of BRAFV600 mutation and pterygium recurrence in the groups: in the groups with traditional surgical methods, the recurrence rate in BRAFV600 mutation-positive patients varied from 73.3% to 81.2%, whereas the recurrence rate in BRAFV600 mutation-negative patients varied from 4.2% to 10.7% (p < 0.001). The mutation increased the risk of a recurrence within a year after pterygium surgery approximately 35.7-fold (OR=35.72; 95% CI, 15.71-81.24). Changing from traditional surgical methods to our methodology for pterygium decreased the recurrence rate at 1 year after pterygium surgery in BRAFV600 mutation-positive patients by 39.8% (or 2.1-fold), from 77.3% to 37.5% (χ2 = 116.92; р < 0.01). Statistical analysis found no relationship between the distribution of recurrences among the groups, and presence of either individual viruses or their combinations (p > 0.1 for all cases). Conclusion: The presence of HSV, EBV, CMV, HPV, and/or BRAFV600 mutation in pterygium tissue is a risk factor for recurrence after surgical treatment. Keywords: pterygium, HSV, EBV, CMV, HPV, BRAFV600 mutation
Introduction Pterygium recurs, most commonly, during the first 4-6 months after surgery, and 97% of recurrences occur within the first year [1, 2, 3]. The above recent meta-analyses have summarized the effects of several adjuvant treatments (conjunctival autograft, mitomycin C (MMC), ciclosporin A, autologous blood, anti-VEGF agents, beta therapy and their combinations) for pterygium. Fonseca et al [1] have found the combination of conjunctival autograft and ciclosporin to be the best treatment to prevent recurrence after primary pterygium surgery, whereas others found intraoperative MMC to be the best adjuvant treatment. However, overall recurrence rates remain rather high (11-38%) [1, 2]. Previously, we have reported on recurrence rates after various surgical techniques for pterygium at immediate (1-3 months) and up to 12 months follow-up points [4]. A number of studies [5, 6] pointed to the etiological role of human papillomavirus (HPV) that triggers the mechanism of excessive cell proliferation. The disease has been found to be associated also with other oncogenic viruses like herpes simplex virus (HSV), Epstein Barr virus (EBV), and cytomegalovirus (CMV) [7, 8, 9, 10]. The BRAF gene is a major factor for activation of epithelial cell proliferation, and codes for the protein that is involved in the signal transfer from membrane tyrosine kinase receptors to the nucleus [11]. The RAF kinase family consists of ARAF, BRAF and CRAF; normally, CRAF has a dominant role, whereas BRAF has been demonstrated to have a dominant role in a number of tumors [12]. Mutated BRAF relays signals to mitogen-activated protein kinase (MEK) and extracellular signal-regulated kinase (ERK), both kinases playing a pivotal role in initiating cell division. The predominant mutation in the BRAF gene involves thymidine to adenosine transversion at exon 15 nucleotide 1799 (T1799>A), resulting in replacement of valine with glutamic acid at position 600 of amino acid sequence (BRAFV600E) [11, 12]. BRAFV600E was found in 87% of papillary thyroid carcinomas, 50% of melanomas, and colorectal cancer [13, 14, 15]. Therefore, this study attempted to examine the relationship between (a) the presence of herpes and papilloma viruses, and BRAFV600E mutation and (b) recurrence rates after various surgical techniques for pterygium. The purpose of this study was to identify the relationship between between (a) recurrence rates after various surgical techniques for pterygium and (b) the presence of herpes viruses (HSV, EBV, and CMV), HPV 6, 11, 16 and 18 and BRAFV600E mutation. Materials and Methods The clinical study was conducted at the Kyiv Municipal Clinical Eye Hospital “Eye Microsurgery Center”, which is a clinical site of the Department of Ophthalmology at the Shupik National Medical Academy of Postgraduate Education. Two hundred and three patients (232 eyes; 108 men and 95 women; age, 35 to 65 years) with a history of pterygium surgery underwent examination. The mean disease duration ranged from 2.5 to 2.7 years. Patients were divided into five groups based on the method of surgical treatment: Group 1 (the McReynolds transplantation alone) of 49 eyes (22.0%); Group 2 (the McReynolds transplantation, with adjuvant MMC 0.02% applied to the cornea for 30 sec) of 41 eyes (17.7%); Group 3 (the technique of Arlt alone) of 49 eyes (21.1%); Group 4 (the technique of Arlt, with adjuvant MMC 0.02% applied to the cornea for 30 sec) of 46 eyes (19.8%); Group 5 of 47 eyes (20.3%) that were treated with our technique of pterygium excision involving the growth plate, with conjunctival autoplasty and adjuvant MMC 0.02% [17]. The groups were statistically similar regarding age, sex, and pterygium stage. All surgeries were performed under local anesthesia by the same surgical team. Pterygium tissue samples were collected during surgery, placed in containers with saline, marked and stored frozen at -70оС until used for DNA extraction. The PROBA-GS DNA Extraction kit (DNK Tekhnologiya, Russia) was used for viral DNA extraction. Real-time PCR with PCR kits from DNK Tekhnologiya were used to identify HSV, EBV, CMV, and HPV. PCR amplification was performed in a DT-lite real-time PCR system (DNK Tekhnologiya). Genomic DNA was extracted and purified using PureLink Genomic DNA kits (Invitrogen, USA). Real-time PCR was used to detect somatic BRAF mutations at rs113488022 (V600E). Mutation detection was performed using TaqMan Mutation Detection Assays (Thermo Fisher Scientific) in a Real-Time PCR System 7500 (Applied Biosystems, USA). Statistical analysis was performed using MedStat and MedCalc v.15.1 (MedCalc Software bvba). Results and Discussion Table 1 shows distribution of patients among groups, recurrence rates at 1 year and rates of BRAFV600 mutations in groups. The cumulative recurrence rate and cumulative rate of BRAFV600 mutations were 28.5% and 35.5%, respectively. There was no significant difference in recurrence rate (χ2=1.32; р=0.723) or rate of BRAFV600 mutations (χ2=1.58; р=0.664) among groups 1, 2, 3 and 4.
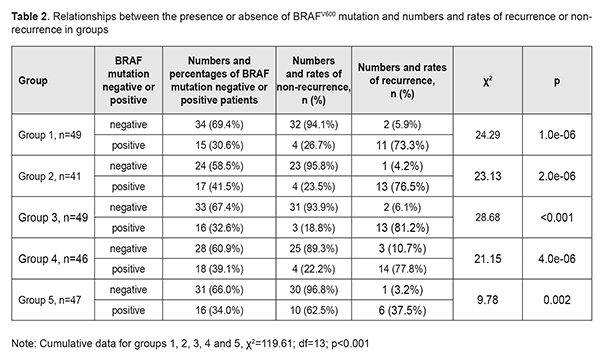
The results of group 5, however, were substantially different from the results of the first four groups. The rate of BRAFV600 mutations in group 5 was similar to (χ2=0.04; р=0.834), but the recurrence rate was markedly lower than those in other groups (14.9% vs 31.9%), i.e. 2.1-fold lower (χ2=5.32; р=0.021). Since we have previously demonstrated an association between BRAFV600 mutations and the incidence of pterygium, we examined numbers and rates of pterigium recurrences and BRAFV600 mutations in individual study groups (Table 2).
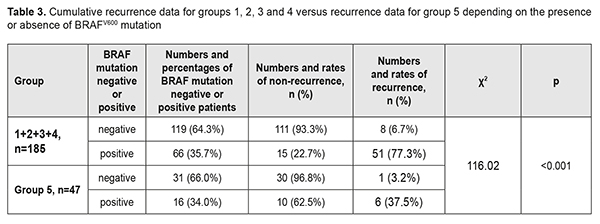
There was a common trend regarding the relationship between the presence of BRAFV600 mutation and pterygium recurrence in the groups. In group 1, of the 34 patients (69.4%) negative for BRAFV600 mutations, 2 (5.9%) had a recurrence, and of the 15 patients (30.6%) positive for BRAFV600 mutations, 11 (30.6%) had a recurrence. Such a difference was statistically significant for all groups (p<0.01 for each group). Thus, the recurrence rate in BRAFV600 mutation-positive patients was 76.5% compared with 4.2% in BRAFV600 mutation-negative patients in group 2, 81.2% vs 6.1% in group 3, and 77.8% vs 10.7% in group 4. In addition, the same trend, but with somewhat different rates of recurrence was observed in Group 5: of the 31 patients (66.0%) negative for BRAFV600 mutations, only one (3.2%) had a recurrence, and of the 16 patients (34.0%) positive for BRAFV600 mutations, 6 (37.5%) had a recurrence. Therefore, in the presence of BRAFV600 mutation, compared to the mean recurrence rate in the four groups related to traditional surgical techniques, the recurrence rate in group 5 related to the pterygium surgery technique developed by us were decreased from 77.3% to 37.5% (χ2=116.02; р<0.001), i.e., by 39.8%, or 2.1-fold (Table 3). The obtained results allowed for the calculation of (a) the effect of BRAFV600 mutation on recurrence rate at 1 year after pterygium surgery and (b) the association of BRAFV600 mutation with the disease (Table 4).
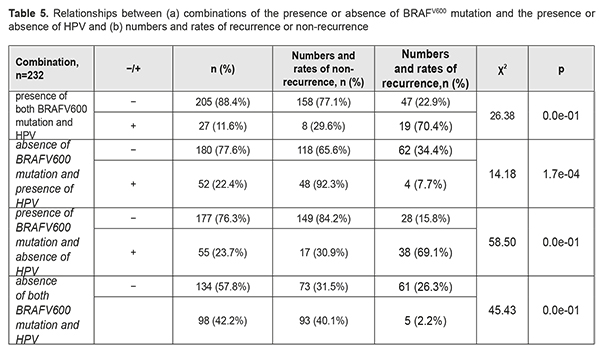
We found BRAFV600 mutation to be associated with a recurrence within a year after pterygium surgery (χ2=105.06; p<0.001). The mutation increased the risk of a recurrence within a year after pterygium surgery approximately 35.7-fold (OR=35.72; 95% CI, 15.71-81.24). Next, we calculated the effect of HSV, EBV, CMV, and HPV 6/11 and 16/ 18 on the development of recurrent pterygium within a year after pterygium surgery. Statistical analysis found no relationship between the distribution of recurrences among the groups, and presence of either individual viruses or their combinations (p>0.1 for all cases). Since it is possible that HPV may act synergistically with oncogenic mutations [5, 6], we tried to compare the distributions of recurrences among the cases with four possible combinations of the presence or absence of HPV (-/+) and the presence or absence of BRAFV600 mutation (Table 5). Of the cases with four possible combinations, the cases with the presence of BRAFV600 in the presence of HPV, and the cases with the presence of BRAFV600 in the absence of HPV, demonstrated the highest recurrence rates (70.4% and 69.1%, respectively), whereas the cases with the absence of BRAFV600 in the presence of HPV, and the cases with the absence of BRAFV600 in the absence of HPV, demonstrated low recurrence rates (7.7% and 2.2%, respectively). These results provide evidence that the presence of HPV infection in pterygium has no effect on the recurrence rate within a year after pterygium surgery.
A study on the possible association of HPV infection and pterygium [6] has reported that the former is not a compulsory etiologic factor but a co-factor increasing the risk of the latter. In the current study, pterygium tissue was found to be infected with a virus in 50.9% of cases, which included HSV, EBV or CMV in 33.6% of cases, and HPV in 34.0% of cases. In addition, HPV 6 and 11 were found mostly in the pterygium stage I or II , and were not found in pterygium stage IV, whereas HPV 16 and 18 were not found in pterygium stage I, and were most common in pterygium stage IV (р=7.95Е-4), which indicates a possible association between HPV and the formation of pterygium. Detorakis et al [16] proposed a two-hit process of pterygium formation. The first hit is preexisting ultraviolet-related genetic damage, and the second hit is mediated by a viral agent [16]. Our study has demonstrated that pterigium formation may represent a three-hit process: in addition to etiologic impact of exogenous factors (ultraviolet exposure and viruses), there is an impact of an acquired endogenous factor, somatic prooncogenic BRAFV600 mutation, with the latter factor having a crucial role in the development of recurrence. We found that, compared with non-carriers, BRAFV600 mutation carriers had an 8-fold increased risk of developing a recurrence during a year after pterygium surgery. Conclusion First, changing from traditional surgical methods (the McReynolds or Arlt method, alone or with adjunctive mitomycin C) to our methodology for pterygium decreased the recurrence rate at 1 year after pterygium surgery by 17.0% (or 2.1-fold), from 31.9% to 14.9% (χ2=5.32; р=0.021). Second, there was a common trend regarding the relationship between the presence of BRAFV600 mutation and pterygium recurrence in the groups: in the groups with traditional surgical methods, the recurrence rate in BRAFV600 mutation-positive patients varied from 73.3% to 81.2%, whereas the recurrence rate in BRAFV600 mutation-negative patients varied from 4.2% to 10.7% (p < 0.001). The mutation increased the risk of a recurrence within a year after pterygium surgery approximately 35.7-fold (OR=35.72; 95% CI, 15.71-81.24). Third, changing from traditional surgical methods to our methodology for pterygium decreased the recurrence rate at 1 year after pterygium surgery in BRAFV600 mutation-positive patients by 39.8% (or 2.1-fold), from 77.3% to 37.5% (χ2=116.92; р < 0.01). Finally, the presence of HSV, EBV, CMV, HPV, or their combinations in pterygium tissue is a risk factor for recurrence after surgical treatment. References 1.Fonseca EC, Rocha EM, Arruda GV. Comparison among adjuvant treatments for primary pterygium: a network meta-analysis. Br J Ophthalmol. 2018 Jun;102(6):748-756. Epub 2017 Nov 16. 2.Zhang Q, Bao N, Liang K, Tao L. Adjuvant Use of Cyclosporine A in the Treatment of Primary Pterygium: A Systematic Review and Meta-Analysis. Cornea. 2018 Aug;37(8):1000-7. 3.Zein H, Ismail A, Abdelmongy M, Elsherif S, Hassanen A, Muhammad B, Assaf F, Elsehili A, Negida A, Yamane S, Abdel-Daim MM, Kadonosono K. Autologous blood for conjunctival autograft fixation in primary pterygium surgery: A systematic review and meta-analysis. Curr Pharm Des. 2018 Oct 1. 4.Rykov SO, Mogilevskyy SIu, Usenko EA. [Recurrence after various surgical techniques for pterygium: 1 year follow-up]. Pytannia eksperymentalnoi ta klinichnoi medytsyny. 2014;18(3):98-102. Ukrainian. 5.Gallagher M, Giannoudis A, Herrington C, Hiscott P. Human papillomavirus in pterygium. Br J Ophthalmol. 2001;85(7):782–4. 6.Piecyk-Sidor M, Polz-Dacewicz M, Zagórski Z, Zarnowski T. Occurrence of human papillomavirus in pterygia. Acta Ophthalmol. 2009; 87 (8): 890–5. 7.Chalkia AK, Spandidos DA, Detorakis ET. Viral involvement in the pathogenesis and clinical features of ophthalmic pterygium (Review). Int J Mol Med. 2013 Sep;32(3):539-43. 8.Otlu B, Emre S, Turkcuoglu P, Doganay S, Durmaz R. Investigation of human papillomavirus and Epstein-Barr virus DNAs in pterygium tissue. Eur J Ophthalmol. 2009 Mar-Apr;19(2):175-9. 9.Rykov SO, Mogilevskyy SIu, Usenko EA. [Impact of HPV and HSV on recurrence after pterygium surgery]. In: [Filatov Memorial Lectures 2016]. Odesa; 2016. p.31-2. Russian. 10.Rykov SO, Usenko KO, Ziablitsev SV, Mogilevskyy SIu. [A method for diagnosing a recurrence in pterygium surgery]. In: [One should be able to see his/her own childhood. Proceedings of the 7th Ukrainian Pediatric Ophthalmology Conference]. July 14-15, 2018; Kyiv. p. 124-6. Ukrainian. 11.Liu R, Zhang T, Zhu G, Xing M. Regulation of mutant TERT by BRAF V600E/MAP kinase pathway through FOS/GABP in human cancer. Nat Commun. 2018 Feb 8;9(1):579. 12.Vuong HG, Duong UN, Altibi AM, Ngo HT, Pham TQ, Tran HM, Gandolfi G, Hassell L. A meta-analysis of prognostic roles of molecular markers in papillary thyroid carcinoma. Endocr Connect. 2017 Apr;6(3):R8-R17. 13.Song JY, Sun SR, Dong F, Huang T, Wu B, Zhou J. Predictive Value of BRAFV600E Mutation for Lymph Node Metastasis in Papillary Thyroid Cancer: A Meta-analysis. Curr Med Sci. 2018 Oct;38(5):785-97. 14.Valachis A, Ullenhag GJ. Discrepancy in BRAF status among patients with metastatic malignant melanoma: A meta-analysis. Eur J Cancer. 2017 Aug;81:106-15. 15.Yang Y, Wang D, Jin L, Wu G, Bai Z, Wang J, Yao H, Zhang Z. Prognostic value of the combination of microsatellite instability and BRAF mutation in colorectal cancer. Cancer Manag Res. 2018 Sep 26;10:3911-3929. 16.Detorakis ET, Drakonaki EE, Spandidos DA. Molecular genetic alterations and viral presence in ophthalmic pterygium. Int J Mol Med. 2000 Jul;6(1):35-41. 17.Rykov SO, Mogilevskyy SIu, Usenko KO, Ziablitsev SV. [Modifed surgical treatment for pterygium reduces the postoperative recurrence rate]. In: [Proceedings of Refractive Plein-Air-18 Conference]. October 18-19, 2018; Kyiv. p. 88-90. Ukrainian.
|