J.ophthalmol.(Ukraine).2018;6:52-58.
|
http://doi.org/10.31288/oftalmolzh201865258 Received: 09 October 2018; Published on-line: 31 December 2018 Soft-tissue response to synthetic polymer implants made of cross-linked polyurethane and containing a biologically active substance, albucid or dacarbazine, in animals N.A. Galatenko,1 Dr Sc (Chem); D.V. Kulyesh,1 Cand Sc (Chem); A.P. Maletskyi, 2 Dr Sc (Med); O.S. Karpenko, 1 Cand Sc (Chem) 1 Institute for Chemistry of High-Molecular Compounds of the NAS of Ukraine; Kyiv (Ukraine) 2 Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine; Odessa (Ukraine) E-mail: maletskiy@filatov.com.ua TO CITE THIS ARTICLE: Galatenko NA, Kulyesh DV, Maletskyi AP, Karpenko OS. Soft-tissue response to synthetic polymer implants made of cross-linked polyurethane and containing a biologically active substance, albucid or dacarbazine, in animals. J.ophthalmol.(Ukraine).2018;6:52-58. http://doi.org/10.31288/oftalmolzh201865258
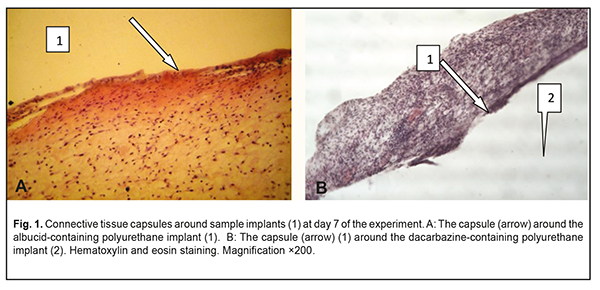
Background: It is an urgent task of the today’s science to search for and introduce biointegrable synthetic materials into medical practice. Purpose: To investigate the soft tissue response to the polymer sample implants made of cross-linked polyurethane with an immobilized biologically active substance, albucid or dacarbazine, in animals. Materials and Methods: Thirty Wistar rats were used to assess the soft tissue response to polymer sample implants under examination. The cross-linked polyurethane-based sample implants with an immobilized biologically active substance, albucid or dacarbazine, were used in the experimental study. Results: The development of the connective tissue capsule around the sample implant, with the capsule completely separating the implant from the surrounding host tissue, was observed at the early time points. Sample implant porosity contributed to cell migration and gradual ingrowth of tissue structures into the implants, which prevented fast implant resorption, and was indicative of the implant biocompatibility with the host tissues. The placement of albucid-containing polyurethane implants in experimental animals resulted in the surrounding host tissue responses typical of aseptic inflammation. The placement of the dacarbazine-containing polyurethane implant in the back of each animal resulted in apparent changes in surrounding host tissue typical for the inflammatory process. It is likely that dacarbazine from the dacarbazine-containing polyurethane sample implant was durably released in the host tissues surrounding the sample, and exerted some biologically active effect which resulted in persistent inflammation at the site of implantation. Conclusion: The albucid- and dacarbazine-containing polymer materials obtained seem promising, and might be widely used as soft tissue substitutes in restorative and reconstructive eye and maxillofacial surgery. Keywords: implant placement, polyurethane, biological activity, soft tissue response, experiment Introduction The rate of ocular, orbital and adnexal trauma has sharply increased in Ukraine in recent decades, with the main contributors to this kind of trauma being industrial accident- or crime-related ocular and/or orbital trauma, intraocular malignant tumors, as well as combat-related ocular injuries characterized by substantial damage to ocular tissues and socket, which are frequently concomitant with traumas to face and other body parts [1-2]. Given the above trend, conducting restorative and reconstructive orbital and periorbital surgeries is important, and the outcomes, if successful, will facilitate the medical, social and professional rehabilitation of patients. These surgeries require the use of graft (or implant) materials for the replacement of soft and bony structures. Presently, a variety of biological and synthetic implant materials are available. Biointegrable implants have an advantage of encouraging ingrowth of recipient’s tissue cells with a reliable implant placement. Biological implant materials include auto, allo- and xenografts. Subcutaneous fatty tissue [3], costal cartilage [4] and bone [5] are common sources for autografts. Allografts come from tissue recovered from qualified deceased human donors. Costal cartilage [6] and lyophilized and/or demineralized bone [7] are commonly used allografts. The use of auricular cartilage allografts [8], fascial lata allografts and scleral allografts [9] has been reported. Xenograft materials, coralline hydroxyapatite [10] and xenopericardium [11] have been proposed for use in orbital surgery. A disadvantage of these materials is that they tend to have problems with resorption. Synthetic implantable materials have been used in medical practice since early 20th century. Methylmethacrylate, polypropylene and polyethylene implants were widely used initially; however, since they are rather rigid and require pre-fabrication, softer and more elastic materials (like silicone and hydrogels) and porous polymer and carbon materials have been developed. Porous polyethylene [12], carbon [13], polytetrafluoroethylene [14], hydrogel [15], ceramics [16], metal [17], coral [18], silicone [19], polyethylene terephthalate [20], corundum ceramic [21], teflon [22], and hydroxyapatite [23-24] implants are presently being used in orbital surgery. Typical disadvantages of synthetic implants include potential dislocation, chemical and mechanical irritation of body tissues, potential toxicity and cost. In addition, potential complications associated with implant-related procedures include infection, exposure, or rejection of the implants. Safe use of synthetic implants in medical practice depends on their capacity for biointegration which is a function of their physical-and-chemical, biological, and immunological characteristics, and largely depends on their spatial pattern. Therefore, it is an urgent task of the today’s science to search for and introduce biointegrable synthetic materials into medical practice for reconstructive and restorative surgery. A promising material for this purpose is a polymer material based on cross-linked polyurethane [25] which contains an urethane group (–NHCOO–) in the polymer chain, and the group is structurally close to the peptide group of proteins –СОNH–, which facilitates effective use of this class of synthetic materials. Polyurethanes are characterized by a heterogeneous structure, which is important for biocompatibility, and, therefore, this class of polymers seems to be promising for biomedical applications. Polyurethanes vary in their chemical structure. Their chemical structure, physical/mechanical properties, and biocompatibility can be widely controlled by varying the synthesis components and conditions [26-31]. Providing biological activity to the polyurethane matrix may be crucial for polymer implant biointegration into pathologically altered tissue [32]. The purpose of this study was to investigate experimentally the soft tissue response to the placement of the implant made of a polymer material based on cross-linked polyurethane with immobilized biologically active substances (albucid or dacarbazine). Materials and Methods The polymer basis, oligoester-urethane diisocyanate, was used as a polymer matrix. Immobilization of a biologically active substance, sulfacyl natrium (albucid) or dacarbazine, was performed to provide biological activity to the polymer material. Albucid is an antibacterial agent used in the prevention and treatment of ocular inflammatory and infectious diseases like conjunctivitis, keratitis, blepharitis, etc. In addition, it is widely used postoperatively to prevent further development of infection. Dacarbazine is an alkylating cytostatic agent and a triazene compound whose mechanism of action is due to the ability of its major metabolite, diazomethane, to form covalent alkyl bonds with the molecules containing electron centers (e.g., -SH groups). Since dacarbazine is structurally similar to purine bases, it acts as an antimetabolite, inhibiting DNA synthesis in tumor cells. We obtained a number of polyurethane composites containing biologically active substances, albucid (1 weight percent) and dacarbazine (1 weight percent). The polymer composites were obtained by sequential mixing of oligoester-urethane diisocyanate, biologically active substances, and a polymerization catalyst at room temperature. The resulting mixture was poured into Teflon molds, and dried in the oven at a constant temperature. Solidified polyurethane composites were porous and elastic. Thirty Wistar male rats (weight, 220 g) were used to assess (a) the soft tissue response to sample implants under examination, and (b) biocompatibility of these samples. All animal experiments were performed under anesthesia and in compliance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series [33]. Surgical procedures on animals were done under aseptic conditions. The operative field was prepared with chlorhexidine. Thereafter, polymer sample implants (sized 10.0х5.0х5.0 mm) were placed subcutaneously in the back of each animal without additional suture fixation to exclude the influence of suturing on wound healing. At days 7, 14 and 30 postimplantation, rats were euthanized by an overdose of chloroform. Implanted polymer samples were collected with surrounding connective tissue and fixed in 10% buffered formalin solution. This was followed by processing, embedding in paraffin, sectioning to 10-15 μm, mounting and hematoxylin and eosin staining in a routine manner [34]. Histological sections were histologically examined by light microscopy at ×150 and × 200 magnifications. Results The state of postoperative field was examined in the course of the experiment. The epithelial response at the operative site was visually assessed daily, and it was found that the wound healed without signs of inflammatory response within three days after intervention. Morphological signs of degenerative changes, tumors, or tissue necrosis were observed neither in the early nor in the late postoperative period. Implanted materials were readily palpated through the skin throughout the period of the experiment. There was no aggression or behavioral changes in experimental animals after implantation of polymer samples. At day 7 after implantation, light microscopy found disintegration of the implanted material from the surrounding tissue due to fibrovascular encapsulation with regard to albucid- or dacarbazine-containing polymer sample implants, with the capsule made up mostly of neutrophils and lymphocytes. At some sites of the capsule surrounding the albucid-containing sample implant, the development of proliferative process due to the active production of extracellular matrix components and collagen fibrils by fibroblast cells was observed (Fig. 1a). In addition, young fibroblastic elements without definite signs of maturity and low differentiated cells were seen at this time point. The blood vessels around the albucid-containing sample implant were few in number and had no pathological changes. At day 7 after implantation of the dacarbazine-containing polyurethane samples, an apparent cellular response was observed, with numerous neutrophils, lymphocytes and monocytes making up a rather wide bank that separated the sample implant from the surrounding tissue (Fig. 1b). The blood vessels around the dacarbazine-containing polymer sample were few in number and characterized by normal microcirculatory processes.
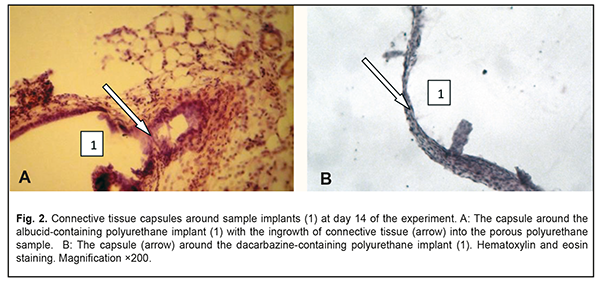
At day 14, a completely developed and mature connective tissue capsule was observed around the albucid-containing polyurethane samples; the capsule was made up of collagen fiber bundles separated by spindle-shaped fibroblasts and oriented along the surface of the sample. There was ingrowth of connective tissue into the porous polyurethane sample at some locations (Fig. 2a), with adjacent residual infiltration with round cells (mostly, neutrophils and macrophages, and, rarely, lymphocytes). Compared to the previous time point, there was an increase in the number of blood vessels around the albucid-containing polymer sample, with some changes in blood microcirculation, and complications in circulation through some microvessels. At day 14, a connective tissue capsule with variations in maturity along its length was observed around the dacarbazine-containing polyurethane samples (Fig. 2b).
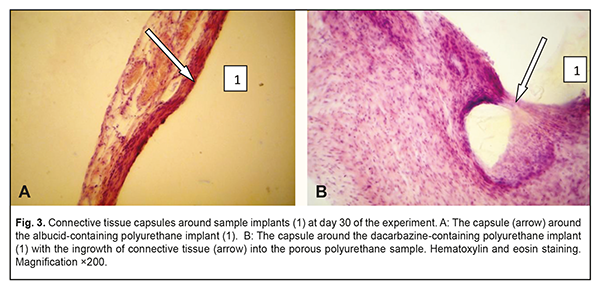
At day 30 time point, similarly to day 14, a thin and mature connective tissue capsule was observed around the albucid-containing polyurethane samples; the capsule was made up of collagen fiber bundles separated by mature spindle-shaped fibroblasts and oriented along the surface of the sample (Fig. 3a). The capsule thickness was somewhat increased due to the proliferation of fibroblastic elements and active production of extracellular matrix components and collagen fibrils by these elements. There were numerous macrophages in some locations of the connective tissue surrounding the implant, which may be explained by an increase in their phagocytic activity. In addition, blood vessels substantially increased in number, and some of them were engorged and dilated. At day 30, an increased inflammatory response with numerous neutrophils was observed in the connective tissue capsule surrounding the dacarbazine-containing, but not albucid-containing polyurethane sample implants. Inflammatory macrophage reaction was also apparent at this time point. There was ingrowth of connective tissue into the porous polyurethane sample at some locations (Fig. 3b), whereas the adjacent tissues were slightly edematous and exudative, which was indicative of an increased inflammation at the site of implantation. The blood vessels did not substantially increase in number compared to the previous time point, and they were characterized by non-pathological microcirculation.
Therefore, in the current study, we demonstrated that the porous structure of implantable polyurethane-based materials with biological activity contributed to the potential of the cells of adjacent tissues for a gradual ingrowth in and biointegration with the samples under examination, providing for a reliable implant fixation at the site of placement. Cell responses to implantation of albucid- versus dacarbazine-containing polyurethane samples in the tissues of experimental animals were typical responses of the animal or human body to the presence of a foreign body at the site of implant placement, but differed from each other. Development and maturation of the connective tissue capsule around the albucid-containing polyurethane sample implant was observed as early as the earliest time points of the experimental study. The development of the connective tissue capsule around the polyurethane sample implant was a natural, biologically determined and predictable process of host response to the presence of sample implant in the body. Throughout the period of the experiment, the cellular responses to albucid-containing polyurethane sample implant were minimal and typical of aseptic inflammation. Connective tissue capsules gradually matured during the period of the experiment, were completely developed, and completely separated the sample implants from the unchanged connective tissue of the host. The capsules were made up mostly of wave-shaped collagen fibril bundles separated by spindle-shaped fibroblasts actively producing collagen fibers and other extracellular matrix components. With regard to the placement of dacarbazine-containing polyurethane sample implants in the tissues of experimental animals, inflammatory cells were highly reactive. Apparent infiltration with neutrophils and macrophage response around the sample implant were observed from day 7. A wide bank made up by white blood cells separated the sample implant from the host connective tissue throughout the period of experiment, with the maximum increase in bank size observed at day 30. An increased response of neutrophils and macrophages to dacarbazine-containing polyurethane sample implants resulted in slight edema and the development of exudate in the connective tissue surrounding the implant. It is likely that dacarbazine from the dacarbazine-containing polyurethane sample implant (a) was durably released in the tissues surrounding the sample, and (b) exerted some biologically active effect which resulted in persistent inflammation at the site of implant placement. This feature of the sample implant may be beneficial in restorative and reconstructive surgeries after removal of a malignant tumor. Conclusion First, elastic, high-density and porous implantable polyurethane-based materials with biological activity were developed. Second, the development of the connective tissue capsule around the polyurethane sample implant, with the capsule completely separating the implant from the surrounding tissue, was observed as early as the earliest time points of the experimental study. In addition, sample implant porosity contributed to cell migration and gradual ingrowth of tissue structures into the implants, which prevented fast implant resorption, and was indicative of the implant biocompatibility with the host tissues. Third, it was found that (a) the albucid-containing polyurethane implantable materials obtained were non-toxic and biocompatible, and (b) their placement in the bodies of experimental animals resulted in the development of cellular responses typical of aseptic inflammation. Fourth, it was found that the inflammatory cells around the sample implant demonstrated high reactivity throughout the period of the experiment. It is likely that dacarbazine from the dacarbazine-containing polyurethane sample implant was durably released in the host tissues surrounding the sample, and exerted some biologically active effect which resulted in persistent inflammation at the site of implant placement. This feature of the sample implant may be beneficial in restorative and reconstructive surgeries after removal of a malignant tumor. Finally, the materials obtained seem promising, and might be widely used as soft tissue substitutes in restorative and reconstructive eye or maxillofacial surgery. However, further research is warranted for conclusive evidence.
References 1.Krasnovid TA. [Ocular trauma under present conditions. Providing urgent care in Ukraine]. Proceedings of the Conference of Ophthalmologists of Chernihiv, Kyiv, and other regions. Chernihiv; 2013. pp. 40-4. Russian. 2.Tselomudryi AI, Venger GE, Pogorelyi DN, Rizvaniuk AV. [Current system of stage-by-stage treatment for combat-related eye injuries in the area of ATO]. Visnyk morskoi meditsyny. 2016;2(71):196-203. Russian. 3.Hintschich C. [Dermis-fat graft. Possibilities and limitations]. Ophthalmologe. 2003 Jul;100(7):518-24. 4.Danz W Sr. Mobility implants: a review. Adv Ophthalm Plast Reconstr Surg. 1990;8:46-52. 5.Karaian AS. [One-stage repair of traumatic defects and deformations of the chhekbone, nose, and orbit complex]. [Dr Sc (Med) Dissertation]. Moscow: Central Research Institute for Dentistry and Maxillofacial Surgery; 2015. Russian. 6.Kugoeva EE. [Diagnosis and management of injuries and diseases of the eyelid and orbit as those of the adnexal structures]. [Dr Sc (Med) Dissertation]. Moscow: Research Institute of Eye Diseases; 1997. Russian. 7.Gorbunova ED. [Clinical picture, diagnosis and treatment of orbital wall fractures in children]. [Cand Sc (Med) Thesis]. Moscow: Research Institute of Eye Diseases; 2006. Russian. 8.Constantian MB. Use of auricular cartilage in orbital floor reconstruction. Plast Reconstr Surg. 1982 Jun;69(6):951-5. 9.Adenis P. [Secondary reconstruction of anophthalmic orbits by intraorbital biomaterial implantation]. J Fr Ophtalmol. 1999 Mar;22(2):269-73. French. 10.Holmes RE. Bone regeneration within a coralline hydroxyapatite implant. Plast Reconstr Surg. 1979 May;63(5):626-33. 11.Grusha IaO, Fedorov AA, Dzemeshkevich VV, Blinova IV. [The clinical-and-morphological specificity of using xenopericardium in plasty of the eyelid and orbit]. Vestn Oftalmol. 2004 Sep-Oct;120(5):19-21. Russian. 12.Froddel J, Seung L. The use of high-density polyethylene implants in facial deformities. Arch Otolaryngol Head and Neck Surg. 1998 Nov;124(11):1219-23. 13.Gundorova RA, Bykov VP, Verigo EN, et al. [On the use of carbon implants in ocular plastic surgery]. Oftalmol Zh. 1996;2:77-9. Russian. 14.Astakhov YuS, Nikolaenko VP, D’yakov VE. [Use of polytetrafluoroethylene implants in ophthalmic surgery]. St Petersburg: Foliant; 2007. Russian. 15.Davydov DV. [Medical and biological aspects of the comprehensive use of biological materials in patients with anophthalmos]. [Abstract of Dr Sc (Med) Dissertation]. Moscow: Research Institute of Eye Diseases; 2000. Russian. 16.Krasilnikova VL. [Foamed ceramics- and l hydroxyapatite-based ocomotor stump of the ocular prosthesis (experimental study)]. [Cand Sc (Med) Thesis]. St Petersburg: Russian Medical Academy of Postgraduate Education; 2002. Russian. 17.Sisler HA, Walsh JB, Finlay JR. Implant with postoperative drain after evisceration. Am J Ophthalmol. 1973 Oct;76(4):537-9. 18.Ferrone PI, Dutton JJ. Rate of vascularization of coralline hydroxyapatite ocular implants. Ophthalmology. 1992 Mar;99(3):376-9. 19.Roze GE. The volume-deficient orbit: Clinical characteristics, surgical management and results after extraperiorbital implantation of Silastic block. Brit J Ophthal. 1990 Sep;74(9):545-50. 20.Kanyukov VN., Stadnikov AA, Trubina OO. [Plastic material for ophthalmic surgery]. Proceedings of the 4th Ophthalmology Conference. Kyiv; 1998: 171-2. Russian. 21.Shatilova TA, Dumbadze GG, Mikadzhe GS, Onikani OV. [Alumina ceramics-based ocular implant]. In: [Plastic surgery of orbit and eye prosthetics]. Collection of scientific works. Moscow; 1981. p. 68-9. Russian. 22.Filatov LN. [The use of Teflon insert and homosclera as implants to form a locomotor stump after evisceration of the eye]. In: [Plastic surgery of orbit and eye prosthetics]. Collection of scientific works. Moscow; 1981. p. 63-5. Russian. 23.Tabatabaee Z, Mazloumi M, Rajabi MT, et al. Comparison of the exposure rate of wrapped hydroxyapatite (Bio-Eye) versus unwrapped porous polyethylene (Medpor) orbital implants in enucleated patients. Ophthal Plast Reconstr Surg. 2011 Mar-Apr;27(2):114-8. doi: 10.1097/IOP.0b013e3181e9790d. 24.Chao DL, Harbour JW. Hydroxyapatite versus polyethylene orbital implants for patients undergoing enucleation for uveal melanoma. Can J Ophthalmol. 2015;50:151-4. 25.Galatenko NA, Rozhnova RA. [Biologically active polymeric materials for medicine]. Kyiv: Naukova Dumka; 2013. Russian. 26.Galatenko NA., Rozhnova RA, Kebuladze IM, et al. [Study of the biocompatibility of linear polyurethanes for endoprosthetics]. Reports of the National Academy of Sciences of Ukraine. 2002;1: 131-5. Russian. 27.Galatenko NA. [Influence of biologically active polyurethane implants on the processes of reparative regeneration and tissue differentiation]. [Cand Sc (Med) Thesis]. Kyiv; 1997. Ukrainian. 28.Kulyesh DV, Nechaeva LIu, Karpik EN, Grytsenko VP, Galatenko NA, Kebuladze IM. [Study of biocompatibility and bioactivity of polyurethane implantable material with immobilized methyluracil]. Plastychna ta rekonstruktyvna khirurgiia. 2014;3-4:49-58. Ukrainian. 29.Galatenko NA, Rozhnova RA. [Investigation of the influence of prolonged form of amison in the cross-linked polyurethane on the course of processes of reparative regeneration]. Visnyk morphologii. 2009;2: 320-5. Ukrainian. 30.Rozhnova RA, Galatenko NA, Narazhaiko LF. [Study of biodegradation and biocompatibility of lactose-containing polyurethane elastomers]. Reports of the National Academy of Sciences of Ukraine. 2006;2:155-61. Ukrainian. 31.Rozhnova RA, Galatenko NA, Savitskaya OS, et al. [Investigation of the effectiveness of polymeric pharmaceutical forms of NSAIDs based on segmented polyurethane elastomers in vivo]. Polimernyi Zhurnal. 2008;30(3):256-61. Ukrainian. 32.Rozhnova RA, Shilov VV, Galatenko NA. [Structural and morphological studies of biologically active implants with prolonged healing action]. Kompozitsiini polymerni materialy. 2000;22 (2): 146-50. Russian. 33.European convention for the protection of vertebrate animals used for experimental and other scientific purposes. Council of Europe, Strasbourg; 1986. 34.Sarkisov DS, Petrova YuL. [Microscopic technique]. Moscow: Meditsina; 1996. Russian.
|