J.ophthalmol.(Ukraine).2018;6:45-51.
|
http://doi.org/10.31288/oftalmolzh201864551 Received: 11 September 2018; Published on-line: 31 December 2018 Correlation between axial length and anterior chamber depth of the eye and retinal disorders in type 2 diabetic rabbits with myopia Mohammad Abdulhadi, Cand. Med. Sc.; I.N. Mikheytseva, Dr. Biol. Sc.; A.A. Putienko, Dr. Med. Sc.; A.G. Kovalchuk, Cand. Med. Sc.; S.G. Kolomiichuk, a research fellow; T.I. Siroshtanenko, a junior research fellow Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine; Odessa (Ukraine) E-mail: filatovbiochem@ukr.net TO CITE THIS ARTICLE: Mohammad Abdulhadi, Mikheytseva IN, Putienko AA, Kovalchuk AG, Kolomiichuk SG, Siroshtanenko TI. Correlation between axial length and anterior chamber depth of the eye and retinal disorders in type 2 diabetic rabbits with myopia. J.ophthalmol.(Ukraine).2018;6:44-51. http://doi.org/10.31288/oftalmolzh201864551
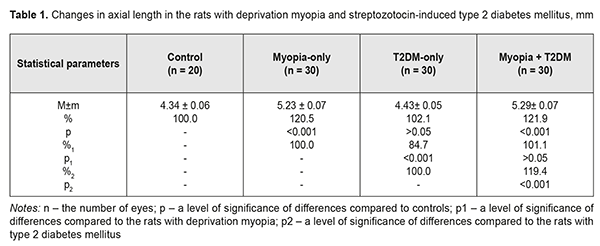
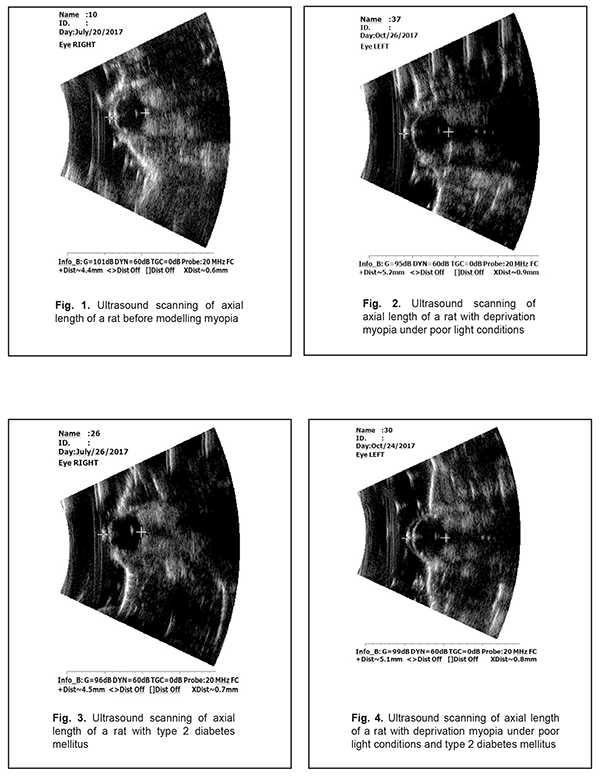
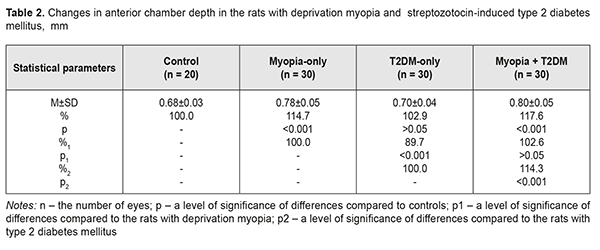
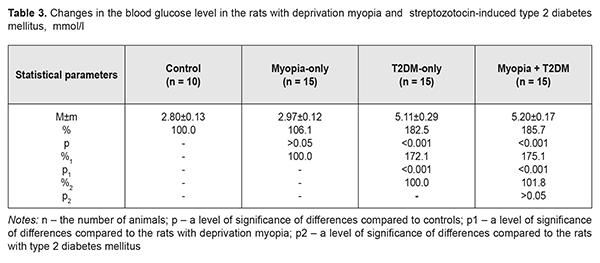
Background. Recently, a protective effect of high myopia on the retina in diabetes has been reported; however, the mechanism of the interrelation between the elongation of axial length and the severity of diabetic complications in the retina is unclear. Purpose. To compare retinal changes in experimental type 2 diabetes melitus (T2DM) in animals with and without myopia as well as to determine the correlation betweeen axial length and anterior chamber depth of the eye and the severity of retinal disorders in DM. Material and Methods. Eyelids of two-week animals (30 rats) were sutured to induce axial myopia [12]. The animals were kept under poor light conditions for 14 days in order to achieve a higher rate of myopia [11, 13]. After a fortnight, the sutures were removed. After another fortnight, 15 rats with experimental myopia and 15 intact rats were modelled T2DM. T2DM was induced using 5 daily intraperitoneal injections of streptozotocin (15.0 mg per 1 kg). Control group comprised 10 intact rats which were kept under natural light condition for 14 days. All the animals were performed ultrasound scanning of the anterior segment to determine axial length (AL) and anterior chamber depth (ACL). A criterion of a diabetes onset was a blood glucose level of 4.5 mmol/l and above. The state of the retina was assessed ophthalmoscopically and expressed using a four-point scoring system. After two months, the animals were sacrificed under general anesthesia and the eyeballs were enucleated. Axial length was measured post mortem using a digital sliding caliper (Topex). Data obtained were processed using the non-parametric tests. Results. In experimental axial myopia, axial length increased by 20.5% compared with control. Experimental type 2 diabetes mellitus did not influence significantly on the axial length of the animals. In the rats with type 2 diabetes and deprivation myopia, axial length was significantly increased by 21.9% and by 19.4% as compared with control and the diabetic-only rats, respectively. Anterior chamber depth in the rats with type 2 diabetes mellitus and deprivation myopia was also increased compared with control. In experimental streptozotocin-induced type 2 diabetes mellitus, the diabetic rats with deprivation myopia had more pronounced changes in the retinal vessels as compared with the non-diabetic rats with deprivation myopia. A negative correlation was revealed between axial length values and the state of the retina in the diabetic rats with deprivation myopia (RSpearman = - 0.68, р<0.05); in a part of the animals, with an increase in axial length, the severity of the pathologic changes in the retina was less pronounced. Conclusion. In our experiment, the myopisation of the eyeball, including the elongation of axial length and the deepening of anterior chamber depth, is accompanied by a decrease of the signs of vascular disorders in the retina which are common for DM. This is also evidenced by the presence of a negative correlation between the myopisation of the eyeball and diabetes-associated disorders in the ocular fundus of the rats. Keywords: deprivation myopia, type 2 diabetes mellitus, retina, ultrasound testing, rats, experiment Background Diabetic retinopathy (DRP) is one of the major causes of vision impairment. Despite the numerous researches worldwide on pathogenesis and treatments for this severe condition, there are still a lot of questions which remain open. In particular, studying the characteristics of a course of different pathological conditions in a single organ is a little-studied and relevant issue. A combination of pathological processes in eye tissues has an effect on pathogenetic mechanisms, especially in cases when pathological processes occur in the same tissue structures and, as a rule, aggravate the course of disease [1]. However, clinical observations have shown a different picture when myopia is combined with diabetes mellitus (DM). In myopia, especially high myopia, the frequency of non-proliferative and proliferative DRP and progression of these diabetic changes in the retina are decreased [2-5]. Meta-analysis of population-based crossover designs has shown that myopia reduces the risk of development of diabetic complications in the retina as compared to emmetropic eyes. Each millimeter increase in axial length in diabetic patients is associated with the decreased incidence of initial and advanced DRP, 0.86 and 0.80 times, respectively. A one diopter increase in refraction and an 11 mm increase in anterior chamber depth are associated with the decreased risk for DR development, 0.9 and 0.32 times, respectively [3]. The correlation between a course of DRP and axial length (AL) of the eyeball has been shown in a clinical study of 104 patients with DM [6]. DRP was approved to progress to a proliferative stage more frequently in patients with AL less than 23.0 mm. AL greater than 24.2 mm provides the rarer and later DRP development. Moss SE et al. have reported that myopia with a refractive error of even -2 diopters can thwart progression of diabetic retinopathy [7]. The presence of myopia was shown to be protective for progression to PDR (non-proliferative to proliferative stages) in younger-onset persons with an odds ratio of 0.40 (95% confidence interval, 0.18–0.86). Based on incidence assessment, it has been concluded that DRP makes up 40.9, 35%, and 70.4 in myopia, emmetropia, and hyperopia, respectively [8]. The authors have reported that no proliferative process is observed in moderate myopia and no DR sings are noted in high myopia. At the same time, Balashevich LI and Izmailova AS have stated that despite the fact of a possible protective effect of a myopic process on a risk for the development and the severity of DRP, proliferative DRP can be developed in patients with extremely high myopia and there are cases when diabetic edema develops in diabetes and myopia with a refractive error of over -20D [1]. In spite of clinical observation data showing that a longer axial length in myopic patients decreases a risk for DRP development [2-5, 9], the mechanism of a protective action of myopia against DRP as well as the association between myopia and structural changes in the eye and in the refractive components is unclear today [2, 10]. Vascular and metabolic factors are highly likely to play a certain role in a decrease of DRP severity in high myopia. This issue of the mechanism of the protective influence of myopia on the severity of diabetic complications in the retina is understudied and waits for to be solved. Studies on the pathogenic mechanisms of diabetic alterations in the retina as well as on the characteristics of structural and functional shifts in DM in the presence of high myopia are needed both to provide scientific rationale for improving prophylaxis and treatment of DRP and to provide a specific practical solution to the problem. To solve this problem successfully, experimental studies using an adequate model of the disease are crucial. Previously, we have published data on advantages of our myopia model in rats [11]. Using that model with higher myopia, we made an effort to compare retinal changes in experimental type 2 DM in animals with and without myopia as well as to determine the correlation between axial length and anterior chamber depth of the eye and the severity of retinal disorders in DM, which was the purpose of the present study. Material and Methods The studies were performed on Wistar rats and followed the General Ethical Principles of Animal Experiments (Third National Congress on Bioethics, Ukraine, Kyiv, 2007) and European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Strasbourg, 1986). Eyelids of two-week animals (30 rats) were sutured to induce axial myopia [12]. The animals were kept under poor light conditions for 14 days in order to achieve a higher rate of myopia [11, 13]. After a fortnight, the sutures were removed. After another fortnight, 15 rats with experimental myopia and 15 intact rats were modelled type 2 diabetes mellitus (T2DM). T2DM was induced using 5 daily intraperitoneal injections of streptozotocin (15.0 mg per 1 kg). Control group comprised 10 intact rats which were kept under natural light condition for 14 days. Thus, we had 4 experimental groups: group 1, 15 animals (30 eyes), the rats with axial myopia (AM-only); group 2, 15 animals (30 eyes), the rats with diabetes (T2DM-only); group 3, 15 animals (30 eyes), the rats with axial myopia and diabetes (AM+T2DM); and group 4, 10 animals (20 eyes), the intact rats as controls (Control). All the animals, for those with experimental myopia - after the sutures had been removed, were performed ultrasound scanning of the anterior segment to determine axial length (AL) and anterior chamber depth (ACL); a blood glucose level was measured using a ME-DC (Germany) glucometer to diagnose diabetes. The state of the retina was assessed ophthalmoscopically and expressed using a four-point scoring system: 0: norm, normal retinal vessels and blood flow; 1 point (the first severity of disorders): moderate changes in the retina, slightly dilated veins and constricted vessels; 2 points (the second severity of disorders): pronounced changes in the retina, dilated veins and more constricted vessels; 3 points (the third severity of disorders): severe changes in the retina, dilated veins and constricted vessels, partial hemorrhages. Ultrasound scanning of the rats’ eyes was made using Cinescan (Quantel Medical) with a 20 MHz B probe for anterior eye with a focal distance of 11-13 mm. The eyelids of the rats were pulled sideward with fingers and instilled a local anesthetic agent. A 10 mm ultrasound gel portion was put on an open eye. The gel made it possible to place the focus of the probe in the center of a rat’s eye inside a big crystalline lens. An axial B-scanning image clearly visualized contours of the cornea (anterior and posterior contours), anterior chamber, anterior and posterior lens capsule, and the vitreoretinal border region. Caliper positioning in a “frozen” image was made with a step of 0.1 mm and velocity of 1 550 m/s. The eyeball length was measured along the axial length between the anterior corneal contour and the vitreoretinal border region. After two months, the animals were sacrificed under general anesthesia and the eyeballs were enucleated. An objective criterion of myopia development in experiment was the elongation of anterior-posterior dimension (APD) of the eyeball and the enlargement of anterior chamber depth in the experimental rats. To follow up the in vivo ultrasound measurements, axial length was measured post mortem using a digital sliding caliper (Topex) with 0.02 mm accuracy. A criterion of a diabetes onset was an increase in blood glucose level up to 4.5 mmol/L and higher. Data obtained were processed using the non-parametric Kruskall-Wallis and Mann-Whitney tests and the Spearman's correlation coefficient using a software program (Statistica 5.5). The data are presented as Mean± Standard Deviation (M±SD). Results and Discussion The major objective criterion of myopia development was the elongation of AL. Findings of ultrasound studies on AL showed that AL in the rats with experimental axial myopia significantly increased by 20.5% compared with intact rats serving as control (Table 1, Figures 1, 2). T2DM modeling did not influence significantly on AL of the rats: no significant difference was revealed between control and diabetic-only groups (Figure 3). AL in the diabetic-only rats was lesser by 15.3% comparing with the myopic-only rats (р<0.001) (Figure 4). AL in the diabetic rats with deprivation myopia was increased by 21.9% (р<0.001) and 19.4% (р<0.001) compared with controls and the diabetic-only rats, respectively.
To follow up the ultrasound studies, AL was measured post mortem using a digital sliding caliper (Topex). Thus, AL was (4.38±0.28) mm, (5.26±0.38) mm, (4.45±0.28) mm, and (5.32±0.39) mm for the Control, AM-only, T2DM-only, and AM+T2DM groups, respectively. The findings of postmortem measurements did not differ significantly from those of the ultrasound studies of AL. Another parameter in the ultrasound studies, which was a characteristic of myopization in the eye, was anterior chamber depth (Table 2, Figures 1-4). As a rule, ACD is deeper in a myopic eye. Our findings also showed an increase in ACD of the rats’ eyes with axial myopia by 14.7 (р<0.05) as compared with controls (Table 2). In modelling T2DM, ACD almost did not change, having increased insignificantly by 2.9% (р>0.05) comparing with controls. ACD in the T2DM-only rats was significantly increased by 10.3% (р<0,001) as compared with the AM-only rats. In the diabetic rats with deprivation myopia, ACD was increased by 17.6% (р<0.001) and 14.3% (р<0.001) comparing with controls and the diabetic-only rats, respectively.
T2DM was induced using a fivefold intraperitoneal injection of subdiabetic doses of streptozotocin (15.0 mg per 1 kg). The occurrence of diabetes was confirmed by elevated blood glucose levels at Day 7 after the streptozotocin injections had been completed. Comparison of the initial blood glucose level in the rats revealed no significant difference between the groups. So, the blood glucose level at baseline (before the experiment started) and at the end of the experiment was, respectively, (2.95±0.24) mmol/l and (2.80±0.13) mmol/l (р>0.05) for the control group; and, respectively, (2.87±0.18) mmol/l and (2.97±0.12) mmol/l (р>0.05) for the group with experimental myopia. Intermittent administration of small doses of streptozotocin, a diabetes inductor, resulted in moderate hyperglycemia in the rats (Table 3). In the rats with streptozotocin-induced diabetes, the blood glucose level increased by 67.5% (р<0.01) and was equal to (5.11±0.29) mmol/l vs. (3.05±0.19) mmol/l at baseline. In the myopic rats with streptozotocin-induced diabetes, the blood glucose level increased from (2.99±0.19) mmol/l to (5.20±0.17) mmol/l, i.e. by 73.9% (р<0.001).
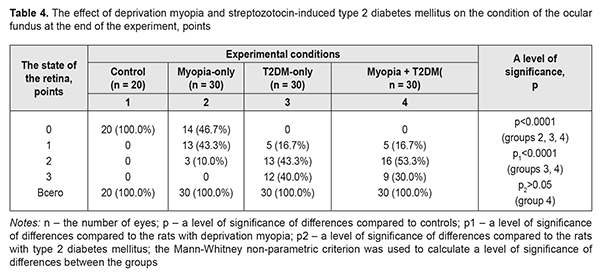
It should be noted that the blood glucose level was significantly increased in the streptozotocin-induced diabetic rats both with and without myopia: by 82.5 and 85.7%, respectively, as compared to controls (р<0.001); and by 72.1 and 75.1%, respectively, as compared to the myopic rats (р<0.001) (Table 3). During the experiment and especially at one month and a half after diabetes induction, the ocular fundus was examined both in the diabetic and non-diabetic rats (Table 4). The retinal vessels in the intact rats (controls) were within the norm throughout the experiment in 100%. Modelling deprivation myopia in the animals led to development of vascular disorders in the retina. In the myopic-only rats: moderate changes in the retina with slightly dilated veins and constricted arteries were noted in 13 eyes (43.3%); more pronounced retinal changes with dilated veins and more constricted arteries were in 3 eyes (10.0%). It should be noted, that retinal vessels were within norm in 14 eyes (46.7%).
In the rats with streptozotocin-induced T2DM, the vascular changes in the retina were more pronounced than those in the rats with deprivation myopia (р1<0.0001): moderate retinal changes with slightly dilated veins and constricted veins in 5 eyes (16.7%); pronounced retinal changes with dilated veins and more constricted arteries in 13 eyes (43.3%); and pronounced retinal changes with dilated veins and constricted arteries and hemorrhage. In this group of the animal, there were no eyes without any vascular changes in the retina. A clinical picture of the ocular fundus in the T2DM rats with myopia tended to be changed (р>0.05) comparing to the retina in the T2DM-only rats (р>0.05). Thus, in the T2DM+myopia group we noted: moderate retinal changes with slightly dilated veins and constricted veins in 5 eyes (16.7%); pronounced retinal changes with dilated veins and more constricted arteries in 16 eyes (53.3%); and severe retinal changes with dilated veins and constricted arteries and sites of hemorrhage. These findings differed significantly from those obtained in the control group and the myopia-only and T2DM-only groups. The ocular fundus of the experimental animals was also assessed ophthalmoscopically using a 0-to-3-score system (Table 5). As we had already mentioned, no changes were revealed in the retina of the intact rats, serving as control, throughout the experiment; the retinal vessels and blood flow were within norm, scoring 0 points.
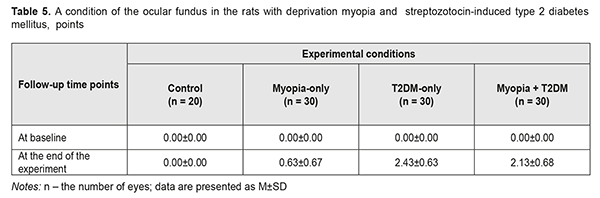
In the animals with myopia, there were changes in the ocular fundus with vascular disorders in the retina in some eyes, which scored (0.63±0.67) points. In T2DM rats, the condition of the retina was worsened and significant retinal changes with dilated veins, constricted arteries, and hemorrhages were revealed, which corresponded to (2.43± 0.63) points. In the eyes with myopia and T2DM, the condition of the retina was some better, scoring (2.13± 0.68) points. However, the changes based on the scoring system were not statistically significant, though the tendency was noted. Thereafter, it should be noted that we revealed a negative correlation dependence between AL and the state of the retina in the myopic rats with T2DM, i.e with an increase in axial length, the severity of pathological changes in the retina was less pronounced in a part of animals. The Spearman's correlation coefficient was equal to R= - 0.68 (р<0.05). The data obtained are in agreement with findings of clinical observations [2, 6]. Assessing the correlation between the blood glucose level and the state of the retina in the T2DM rats with deprivation myopia, a positive correlation was noted: the Spearman's correlation coefficient was equal to R=0.70 (р<0.05). So, according to the findings by Wat N. et al. (2016), a good glycemic condition is an important factor for reducing the risk of progression of diabetic retinopathy [4]. Thus, we revealed a positive correlation between levels of glycemia and the severity of the retinal disorders in the rats. When studying the correlation between the axial length parameters and the severity of retinal changes in the rats with both experimental T2DM and myopia, a statistically significant negative correlation was observed, based on the Spearman's correlation coefficient. It is quite conceivable that a certain role in the “protective” effect of myopia on the severity of retinal changes belongs to alterations in the vitreous body which contribute to the active release of compounds with an oxidant action, including the hyaluronic acid. In addition to these features, there is an idea that the rectification of the ocular vessels in the eye with longer axial length prevent from venous stasis [6, 14] by producing a positive effect on the structural and functional features of the retina. The data obtained give evidence of a need for further investigations with the purpose to reveal pathogenetic mechanisms of the positive effect of myopia on the structure and metabolism of the retina in myopic animals with experimental T2DM. To conclude; firstly, analysis of the data obtained from the streptozotocin-induced diabetic rats with and without axial myopia give evidence that the experimental myopisation of the eyeball, including the elongation of axial length and the deepening of anterior chamber depth, also decreases the signs of vascular disorders in the retina which are common for DM. Secondly, the experimentally-revealed negative correlation between myopia and diabetes-associated disorders in the ocular fundus of the rats will make it possible to determine the pathological mechanisms of this process.
References 1.Balashevich LI, Izmailova AS. [Diabetic Ophthalmology]. S.-Petersburg SPB:Chelovek; 2012. 396p. Russian. 2.Tayyab H, Haider MA, Haider Bukhari Shaheed SA. Axial myopia and its influence on diabetic retinopathy. J. Coll. Physicians Surg. Pak. 2014 Oct;24(10):728-31. doi: 10.2014/JCPSP.728731. 3.Wang X, Tang L, Gao L, Yang Y., Cao D, Li Y. Myopia and diabetic retinopathy: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2016 Jan;111:1-9. doi: 10.1016/j.diabres.2015.10.020. Epub 2015 Oct 23. 4.Wat N, Wong RL, Wong IY. Associations between diabetic retinopathy and systemic risk factors. Hong Kong Med. J. 2016;22( 6):589-99. 5.Bazzazi N., Akbarzadeh S., Yavarikia M., Poorolajal J., Fouladi D.F. High myopia and diabetic retinopathy: A Contralateral Eye Study in Diabetic Patients With High Myopic Anisometropia. Retina. 2017 Jul; 37(7):1270 -1276. doi: 10.1097/IAE.0000000000001335. 6.Bobr T. [Features of the course of diabetic retinopathy depending on the magnitude of the anterior-posterior axis of the eye]. Oftalmologiia. Vostochnaia Evropa. 2017;7(2):152-6. Russian. 7.Moss SE, Klein R, Klein BE. Ocular factors in the incidence and progression of diabetic retinopathy. Ophthalmology. 1994;101(1):77-83. 8.Sultanov MI, Gadzhiev RV. [Features of the course of diabetic retinopathy in myopia]. Vestn Oftalmol. 1990;106(1):49-51. Russian. 9.Man RE, Sasongko MB, Sanmugasundram S, Nicolaou T, Jing X, Wang JJ, Wong TY, Lamoureux EL. Longer axial length is protective of diabetic retinopathy and macular edema. Ophthalmology. 2012:119( 9):1754-9. doi: 10.1016/j.ophtha.2012.03.021. Epub 2012 May 23. 10.Man RE, Sasongko MB, Wang JJ, Lamoureux EL. Association between myopia and diabetic retinopathy: a review of observational findings and potential mechanisms. Clin. Exp. Ophthalmol. 2013 Apr; 41(3):293-301. doi: 10.1111/j.1442-9071.2012.02872.x. Epub 2012 Oct 29. 11.Mikheytseva IN, Mohammad Abdulhadi, Putienko AA, Kovalchuk AG, Kolomiichuk SG, Siroshtanenko TI. Modelling form deprivation myopia in experiment. Journal of Ophthalmology (Ukraine). 2018;2:50-5. 12.Beuerman RW, Maw SS, Tan DT et al. Myopia: animal models to clinical trials. Singapore World Scientific; 2010. 390 p. 13.Mohammad Abdulhadi, Mikheitseva IN, Putienko AA et al. [Rationale for a use of a form deprivation myipia model in studying pathogenesis and treatment of a disease].[Proceedings of scientific and practical conference of ophthalmologists of Chernyvtsi, Ivano-Frankivsk, Ternopil, Khmelnytskyi regions of Ukraine. 20-21 September 2017. Chernyvtsi]. Odessa, Chernyvtsi;2017:60-1. Russian. 14.Bolshunov AV, Kulieva IA. [Characterisics of the clinical course of diabetic retinopathy in myopia]. Vestn Oftalmol. 1998;6:54-6. Russian.
|