J.ophthalmol.(Ukraine).2018;6:40-43.
|
http://doi.org/10.31288/oftalmolzh201864043 Received: 5 November 2018; Published on-line: 31 December 2018 Maxillary postimplantation syndrome: ocular aspects A.O. Asmolova,1 Cand Sc (Med); O.V. Zborovska,2 Dr Sc (Med); O.E. Dorokhova,2 Cand Sc (Med); O.V. Pasechnik,1 Cand Sc (Med) 1 Odesa National Medical University; Odessa (Ukraine) 2 Filatov Institute of Eye Disease and Tissue Therapy; Odessa (Ukraine) E-mail: dorochovaa@gmail.com TO CITE THIS ARTICLE: Asmolova AO, Zborovska OV, Dorokhova OE, Pasechnik OV. Maxillary postimplantation syndrome: ocular aspects. J.ophthalmol.(Ukraine).2018;6:40-43. http://doi.org/10.31288/oftalmolzh201864043
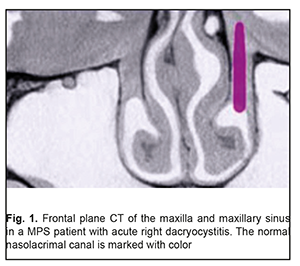
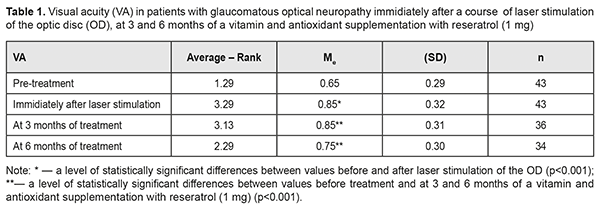
Background: Maxillary postimplantation syndrome (MPS) has been previously described as a complication occurring more than 1 year after maxillary implant placement in patients with partial loss of teeth due to accident, extraction and/or local periodontal disease. The syndrome is characterized by dental, rhinological, ocular and/or neurodental symptoms. Purpose: To determine frequencies of ocular disorders in patients who developed manifestations of maxillary postimplantation syndrome. Materials and Methods: Seventy four patients with MPS (age, 27 to 64 years) and 43 healthy volunteers (age, 25 to 63 years) were involved in the diagnostic study. Patients underwent a routine clinical examination including rhinoscopy, stomatoscopy, and paranasal sinus computed tomography. In addition, a routine eye examination was performed. Results: Ocular disorders were found in 81% of patients with MPS, and included functional injury of the nasolacrimal canal (51%), dacryocystitis (20.2%), and intermediate uveitis (6.7%) with complications manifesting as macular edema or epiretinal membrane (4.05%), optic nerve drusen (6.7%), and optic neuritis (5.4%). Some MPS patients exhibited several ocular disorders (including, in particular, a functional injury of the nasolacrimal canal or dacryocystitis). Conclusion: Ocular disorders were found in 81% of patients with MPS. Patients with MPS should undergo an eye examination even in the absence of ocular complaints. Keywords: dental implant placement, maxillary postimplantation syndrome, uveitis, dacryocystitis, optic nerve drusen Introduction Dental implant placement is an on-demand treatment option for patients with total or partial loss of teeth, and, like any surgery, has potential complications. Although intraoperative and postoperative complications within a year after implant placement have been well studied, some complications occurring more than 1 year after implant placement are poorly studied [1, 2]. It is identification, treatment and prevention of the latter complications that are an urgent problem. Maxillary postimplantation syndrome (MPS) has been previously [1-5] identified as an autonomous clinical entity, a complication occurring 1-5 years after maxillary implant placement in patients with partial loss of teeth due to accident, extraction or local periodontal disease. The syndrome is characterized by dental, rhinological, ocular and/or neurodental symptoms in the presence of pathological environmental afferent activity: bone lamella at least 0.5 mm thick above the implant body end, nasal septum deviation, osteomeatal complex defects or abnormalities, chameprosopic or mesoprosopic facial skeleton, mucociliary dyskinesia, inactive or reduced response to cooling, Misch D3 to D4 alveolar bone density, external carotid artery stenosis, chronic maxillary sinusitis, facial pain, paresthesia of the upper lip, and hyperalgesia in the territory of the second branch of the trigeminal nerve [2, 3, 5]. The purpose of the study was to determine frequencies of ocular disorders in patients who developed manifestations of maxillary postimplantation syndrome. Materials and Methods Seventy four patients with MPS (age, 27 to 64 years) were involved in the diagnostic study. Of these, 44 (59.5%) had received unilateral dental implants, and 30 (40.5%), bilateral dental implants. The control group comprised 43 healthy volunteers (age, 25 to 63 years) without any symptoms of MPS. The inclusion criteria were as follows: at least 12 months after implant placement; presence of left- or right-side implants only in the maxilla; absence of clinical manifestations of endocrine or cardiovascular diseases. Exclusion criteria were less than 12 months after implant placement and taking regular medications for any chronic disease. Patients underwent a routine clinical examination including rhinoscopy, stomatoscopy, and paranasal sinus computed tomography. In addition, a routine eye examination was performed. The study protocol was approved by a local Bioethics Committee of the Odesa Medical University. Patients were fully informed about the diagnostic procedures involved, and signed an informed consent form. Variation statistics were used for data analysis [6]. T test was used for pairwise comparisons, and the level of significance p ≤ 0.01 was assumed. Results and Discussion Seventy four patients (100%) developed specific symptoms of maxillary rhinosinusitis including mucoid nasal discharge, nasal obstruction, and a sensation of pressure or tightness that involved the nose bridge area and was becoming more intense with head flexion. In addition, 55 patients (74.3%) had ocular complaints. Of these, 42 (76.4%) complained mainly of tearing and tear stasis, 5 (9.1%) complained mainly of floaters or clouding of vision (of these 5 patients, 3 complained also of decreased vision), and 8 (14.5%) complained only of decreased vision. Some patients complaining of floaters, clouding of vision and/or decreased vision also complained of tearing and tear stasis. Computed tomography of the maxilla and maxillary sinus provides some imaging also of orbit structures (Fig. 1). Bilateral changes in the maxillary sinuses were observed in all cases with bilateral placement of implants.
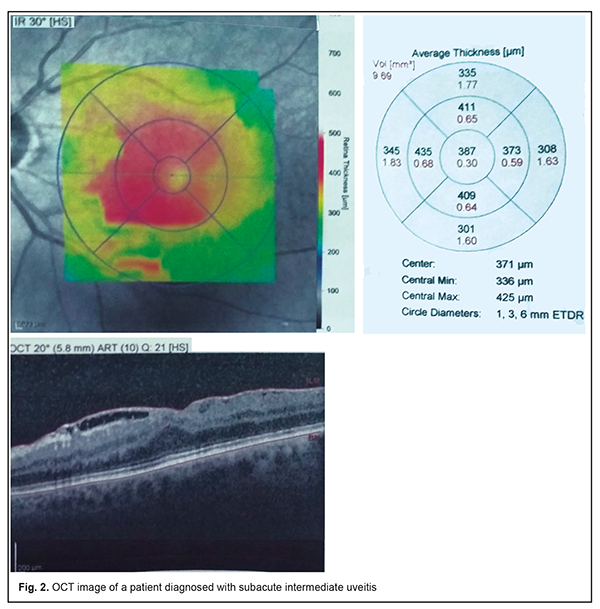
The tear sac was located at the typical anatomical location (i.e., at the level of the anterior end of the middle turbinate bone), posterior to this location, and significantly anterior to this location in only 31 (41.9%), 24 (32.4%), and 19 patients (25.7%), respectively, of the MPS group versus 73.7%, 15.8% and 10.5%, respectively, of the controls, and the differences were statistically significant (p < 0.01). Symptoms of acute unilateral dacryocystitis were observed in 15 (35.7%) of the MPS patients complaining of tearing and tear stasis. These 15 patients all had CT findings of anatomical abnormalities in the maxillary sinus or osteomeatal complex, with 8 patients (53.3%) demonstrating changes in the maxillary sinus and 7 patients (46.7%), in the ethnoid sinuses. In the rest 40 MPS patients (72.7%) complaining of tearing and tear stasis, the pathology was only functional in nature, and likely associated with persistent nasal mucosal swelling including nasolacrimal canal swelling on the affected side. Of these 40 patients, 11 had a negative result of dye disappearance test for assessment of lacrimal passage functional potency, and the rest demonstrated a delay of dye passage of the ocular surface and into the nose. All 5 patients (9.1%) complaining of floaters or clouding of vision were diagnosed with subacute intermediate uveitis. The patients with complaints of both floaters and decreased vision had already developed a posterior segment complication, either macular edema or epiretinal membrane, which was confirmed by optical coherent tomography (Fig. 2). None of the control group was diagnosed with uveitis.
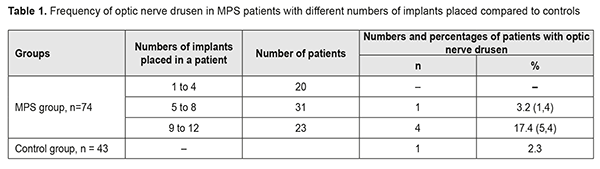
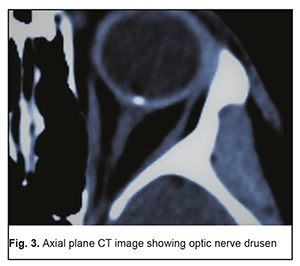
In 2 (18.2%) of the 11 MPS patients with decreased vision, it was due to age-related changes, and these two patients were diagnosed with age-related macular degeneration (AMD). AMD was diagnosed in one patient (2.3%) of the control group. The study found no statistically significant difference in the risk of AMD between patients with MPS and controls. Of the MPS patients with decreased vision, 6 (54.5%) were diagnosed with an optic nerve disorder, including 2 (33.3%) with anterior or posterior ischemic optic neuropathy and 4 (66.7%) with optic neuritis. Only one patient (2.3%) of the control group was diagnosed with an optic nerve disorder, anterior ischemic optic neuropathy that ran a symptomless course. Based on the data obtained, we can state that patients with MPS are at risk of developing optic neuritis. Whether patients with MPS are at risk of developing ischemic optic nerve disease is still an open question and requires further research with larger study cohorts. However, the pattern of blood supply of the carotid artery (CA) in these patients suggests that they are at risk of ischemic neuropathy. Previously, we have found [3] CA injuries in 63.5% of patients with MPS versus 21.1% of the controls; the difference was statistically significant (р < 0.01). Unilateral CA injuries were ipsilateral to the implant side (p < 0.02). Mild, moderate and severe internal carotid artery stenosis in patients with MPS were found 3.47, 2.05 and 4.15 more frequently than in controls, and the difference was statistically significant (p < 0.011). It is noteworthy that, in the current study, optic nerve drusen were observed in 5 (26.3%) of the 19 MPS patients without ocular complaints, or 6.8% of all the patients in the MPS group, versus 1 (2.3%) in the controls. These drusen were easily found on CT since they contain calcium. The numbers and percentages of patients with optic nerve drusen tended to increase with an increase in the number of implants placed (Table 1).
In adults, studies have found a prevalence of optic nerve drusen of 0.5 to 2.4% [7, 9]. A review by Chang and Pineles [9] includes lists of (a) 217 references related to optic nerve drusen and published until December 2015, (b) 26 ocular disorders reported in association with optic disc drusen, and (c) systemic disorders reported in association with optic disc drusen. An article by Fotzsch [10] was referenced in the review as the article reporting an association of optic nerve drusen with teeth and jaw anomalies. In our opinion, optic nerve drusen are a symptom reasonably associated with MPS. In the previous study [1], anatomical abnormalities of the nasal septum, uncinate process, middle turbinate, and ethmoidal bulla, agger nasi cells, Haller cells, extramural frontoethmoidal cells, medial orbital wall dehiscence, agger nasi cell hypertrophy, and accessory maxillary sinus ostium have been seen in 100%, 39.4%, 37.9%, 21.2%, 43.9%, 7.6%, 13.6%, 15.2%, 9.1% and 21.2%, respectively, of patients with MPS, compared with 48.2%, 17.9%, 14.3%, 12.5%, 19.6%, 3.6%, 5.4%, 3.6%, 3.6%, and 7.1%, respectively, in patients without complaints after maxillary dental implant placement. Optic nerve drusen can be inherited or acquired [7, 9]. The etiopthogenetic role [7] of decreased activity of enzymatic systems, effect of aseptic inflammatory responses, development of secondary immune deficiency, and increased free radical oxidation in the development of drusen has been reported [4].
Therefore, ocular disorders were found in 81% of patients with MPS, and included functional injury of the nasolacrimal canal (51%), dacryocystitis (20.2%), and intermediate uveitis (6.7%) with complications manifesting as macular edema or epiretinal membrane (4.05%), optic nerve drusen (6.7%), and optic neuritis (5.4%). Some patients exhibited several ocular disorders (including, in particular, a functional injury of the nasolacrimal canal or dacryocystitis). Our finding of carotid artery stenosis led us to hypothesize that MPS is associated with the development of ischemic optic neuropathy. However, this question is still open and warrants further investigation. In addition, it is noteworthy that, in the presence of an ocular disorder in patients with MPS, they may have either no ocular complaints (e.g., in case of optic nerve drusen), or minor ocular complaints like floaters or clouding of vision in case of a rather serious disorder like intermediate uveitis. Our findings stress the requirement for meticulous eye examination of patients with MPS even in the absence of ocular complaints. Conclusions Ocular disorders were found in 81% of patients with MPS. Patients with MPS should undergo an eye examination even in the absence of ocular complaints. References 1.Asmolova AA. [Maxillary postimplantation syndrome: abnormal variant anatomy of the paranasal sinuses]. Luchevaia diagnostika, luchevaia terapiia. 2015; 3–4:47–53. Russian. 2.Asmolova AA. [Maxillary postimplantation syndrome: clinical observations]. Luchevaia diagnostika, luchevaia terapiia. 2018; 1:78–81. Russian. 3.Asmolova AA. [Maxillary postimplantation syndrome: status of carotid arteries]. Luchevaia diagnostika, luchevaia terapiia. 2016; 1:22–7. Russian. 4.Asmolova AA. [Status of oral fluid in maxillary postimplantation syndrome]. Innovatsii v stomatologii. 2017; 1:11–4. Russian. 5.Аsmolova AА. Dental implants can generate maxillary postimplantation syndrome. Arta Medica. 1(54):28–30. 6.Lapach SN, Chubenko AV, Babych PN. [Statistical methods in biomedical research using Excel]. 2nd ed. Kyiv: Morion; 2001. Ukrainian. 7.Sheremet NL. [Diagnostics of optical neuropathies of different origin]. Dissertation for the degree of Doctor of Medical Science. Moscow: Research Institute for Eye Disease; 2015. Russian. 8.Chang MY, Pineles SL. Optic disc drusen in children. Surv Ophthalmol. 2016 Nov - Dec;61(6):745-58. doi: 10.1016/j.survophthal.2016.03.007. 9.Fotzsch R. Problems in the etiology, pathogenesis and clinical significance of drusen (hyalin bodies) of the optic disk. Psychiatr Neurol Med Psychol (Leipz). 1970 Jun;22(6):223-7. https://doi.org/10.31288/oftalmolzh20185314 Efficacy of morpholinium-methyl-triazolyl-thioacetate (thiotriazolin) for neurotrophic keratoconjunctivitis in long-term silicone hydrogel contact lens wearers T.A. Veliksar; T.B. Gaidamaka, Dr Sc (Med); G.I. Drozhzhina, Dr Sc (Med), Prof.; I.M. Mikheitseva, Dr Sc (Biol); S.G. Kolomiichuk, Research Fellow Received: 23 August 2018 Published: 26 October 2018 Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine; Odesa (Ukraine) E-mail: tveliksar@gmail.com Background: Vision correction with contact lenses offers some clear advantages as compared to correction with spectacles, but has some negative effect on the eye, too. Individuals wearing soft contact lenses (SCL) for years may develop neurotrophic keratoconjunctivitis that requires a long-term and mostly symptomatic treatment. Purpose: To compare the efficacy of different routes of morpholinium-methyl-triazolyl-thioacetate (thiotriazolin) administration for neurotrophic keratoconjunctivitis in patients wearing silicone hydrogel contact lenses for different periods of time. Materials and Methods: Two hundred and seventeen eyes of 112 patients with low or moderate myopia were examined and treated. These included 65 eyes of 33 patients wearing spectacles, and 159 eyes of 79 patients wearing SCL for long periods of time. SCL wearers were divided into subgroups depending on the treatment regimen and categorized depending on duration of SCL wear. Lactate dehydrogenase, glucose-6-phosphate dehydrogenase, succine dehydrogenase, and acid phosphatase activities in the tear film, and tear contents of glutathione, malondialdehyde and diene conjugates were examined. Results and Conclusions: Treatment with thiotriazolin statistically significantly decreased lipid peroxidation activity levels, with a reduced disruption of ocular surface membranes, and protected the ocular surface from oxidative stress. Keywords: neurotrophic keratoconjunctivitis, morpholinium-methyl-triazolyl-thioacetate (thiotriazolin), lactate dehydrogenase, glucose-6-phosphate dehydrogenase, acid phosphatase, succine dehydrogenase, glutathione, malondialdehyde, diene conjugates
Introduction Vision correction with contact lenses offers some advantages as compared to correction with spectacles, but has some negative effect on the eye, too. It has been reported that annually about 0.5% of individuals wearing soft contact lenses (SCL) give up wearing them due to SCL intolerance or discomfort, and 5.2-15% of such individuals develop corneal allergic, toxic, infectious and/or metabolic complications [1-6]. Tissue hypoxia and mechanical compression are considered the most important contact lens-related factors affecting the anterior eye structures. Wearing contact lenses for a long time may lead to abnormalities in eye-winking reflex, tear production, and precorneal tear film stability, which may result in corneal surface roughness. Hypoxia causes metabolic alterations in the corneal epithelium and stroma, whereas direct contact of the lens with the ocular surface may lead to alterations in corneal curvature. Chronic hypoxia of the ocular surface is a leading cause of metabolic alterations in vision correction with contact lenses [7-11]. In addition, corneal metabolic changes are largely mediated by the effect of SCL on the tear film [5]. Normally, tears provide oxygen to the cornea and wash away carbon dioxide. A contact lens provides a barrier to appropriate delivery of oxygen to and removal of products of metabolism from the eye, with the corneal epithelial permeability for carbon dioxide being 7 times higher than that for oxygen. Therefore, a contact lens creates conditions for a change in corneal pH, and, thereby, for an alteration in corneal metabolism. As a result, a shift takes place from aerobic energy production (i.e., glycolysis) towards anaerobic energy production in the epithelium, with a substantial increase in lactic acid production per energy unit. The lactic acid produced becomes accumulated in the outer corneal stroma, and corneal osmolarity becomes higher than that of the surrounding tear film or aqueous humor, with ingress of water from both sides to the cornea resulting in a decrease in stromal tonus. The corneal saturation with water exceeds the capacity for water withdrawal of the corneal endothelial pump that lacks energy for effective performance under anaerobic metabolism conditions [12, 13]. Brzheskiy et al, Nikolska, and Teriokhina et al were among the first developers of tear research methodologies. Brzheskiy et al (1985) developed a methodology to measure tear glucose level. Nikolska (1985) developed a technique to assess coagulation and fibrinolytic activity of tear fluid. Teriokhina et al (1988) developed a method to measure tear enzyme levels in an effort both to predict the course and the chance of recurrence and to assess the efficacy of treatment for herpetic keratitis. Numerous studies have been reported on tear biochemistry in various ocular disorders [12-20]. Tear fluid provides physiological protection for the cornea, provides optimal conditions for various biochemical processes (first of all, for gas exchange between the cornea and atmospheric air) at the corneal surface [21, 22]. A balance between lipid peroxidation (LPO) and antioxidant systems plays an important role in achieving and maintaining adaptation of the body in general and any body structure in particular [13, 19, 20, 23]. Lipid peroxidation is a non-specific process, a lipid response to any stress during cell adaptation to the effect of negative factors [18, 20, 24]. It has been found that activation of LPO results in alteration in the phospholipid content of cell membranes. Elevated lipid peroxidation activity impedes microcirculation and nerve receptor function. In addition, it alters (a) nerve receptor sensitivity to hormones, mediators and immune parameters, and (b) adaptive capacity of cells. The final effect of LPO on tissues, cells and subcellular structures, and the degree of free radical-mediated tissue damage depend on the state of the antioxidant system [16]. Wearing SCL even for two hours causes a shift towards anaerobic metabolism in the cornea, which is evidenced by elevated activity of lactate dehydrogenase (LDH) in the tear film and hypoxic corneal swelling due to enzymatic dysfunction of the endothelial pumps [13]. Biochemical studies of tear film are important in diagnosing various ocular disorders, predicting their course, and assessing the efficacy of therapeutic treatment for them [14-17]. The technique is non-invasive, readily available and provides valuable information about biochemical tear film parameters. Individuals wearing SCL for long periods of time may develop neurotrophic keratoconjunctivitis. The disease requires a long-term treatment that is mostly symptomatic and involves artificial tears and antibacterial and anti-inflammatory agents [25-28]. In stage 1 neurotrophic keratoconjunctivitis, the pathological anterior eye changes may not cause significant complaints and discomfort due to reduced corneal sensitivity, and patients seldom seek medical advice for the disease. Stage 1 neurotrophic keratoconjunctivitis is found mostly during check-ups, but, if untreated, may progress to higher stages with the development of serious complications. We have proposed a method for treating neurotrophic keratoconjunctivitis with morpholinium-methyl-triazolyl-thioacetate (thiotriazolin) using various routes of administration. Pre-treatment studies of biochemical profiles of tear fluid in long-term SCL wearers would improve our knowledge on the pathogenesis of ocular surface changes depending on lens-wearing periods, and the comparison of pre-treatment and post-treatment data would contribute to clarification of the nature and extent of influence exerted by the agent on the pathological changes. The purpose of the study was to compare the efficacy of different routes of administration of morpholinium-methyl-triazolyl-thioacetate (thiotriazolin) for neurotrophic keratoconjunctivitis in patients wearing silicone hydrogel contact lenses for different periods of time. Materials and Methods This study included 217 eyes of 112 patients with low or moderate myopia who were examined and treated at the Department of Corneal Microsurgery, Filatov Institute. The spectacle group (or control group) comprised 65 eyes of 33 patients with low or moderate myopia corrected with spectacles (25 (75.8%) women and 8 (24.2%) men; age, 22 to 51 years; mean age, 29.8±0.9 years), with a mean refraction of -3.87±0.2 D, uncorrected visual acuity (UCVA) of 0.01 to 0.7 (mean UCVA, 0.24±0.03), and best-corrected visual acuity (BCVA) of 0.4 to 1.2 (mean BCVA, 0.88±0.03). The contact lens (CL) group comprised 152 eyes of 79 patients (61 (77.2%) women and 18 (22.8%) men; mean age, 30.8±0.4 years) wearing contact lenses for low or moderate myopia for 1 to 41 years, with a mean refraction of -4.12±0.2 D (p=0.17), mean UCVA of 0.12±0.01 (p=0.0000), and mean BCVA of 0.86±0.02 (p=0.28). Patients of the CL group were divided into three subgroups based on treatment regimen. Patients of subgroup 1 received topical antiseptic (boric acid 2%) 2 times daily, thiotriazolin 1% 4 times daily, preservative-free hyaluronic acid artificial tears (optinol 0.21%) 4-6 times daily, and 1 thiotriazolin tablet 2 times daily for 1 month. Patients of subgroup 2 received topical antiseptic (boric acid 2%) 2 times daily, thiotriazolin 1% 4 times daily, and preservative-free hyaluronic acid artificial tears (optinol 0.21%) 4-6 times daily for 2 weeks. In addition, they received a course of transorbital electrophoresis with thiotriazolin 1% (10 procedures daily). Patients of subgroup 3 received topical antiseptic (boric acid 2%) 2 times daily and preservative-free hyaluronic acid artificial tears (optinol 0.21%) 4-6 times daily for 1 month. Patients of the CL group were also divided into three categories based on CL-wearing period (1 to 5 years; 6 to 10 years; and > 10 years). Subgroups 1, 2 and 3 comprised 33 patients (62 eyes; 24 (72.7%) women and 9 (27.3%) men), 31 patients (62 eyes; 23 (74.2%) women and 8 (25.8%) men), and 15 patients (28 eyes; 14 (93.3%) women and 1 (6.7%) man), respectively. The three subgroups were homogeneous in terms of gender, and women prevailed in all of them (χ2 =2.7; p = 0.25). In addition, these subgroups were age-matched (F=0.42; p=0.66), and mean ages in subgroups 1, 2 and 3 were 30.9±0.82 years, 31.3 3±0.63 years, and 29.7±0.65 years, respectively. Mean refractive errors in subgroups 1, 2 and 3 were -4.22±0.17 D, -4.18±0.16 D, and -3.77±0.29 D, respectively (F=1.20; p=0.31). There was no significant difference in CL-wearing period among the subgroups (F=0.54; p=0.58). In patients of subgroups 1, 2 and 3, mean CL-wearing period was 10.5±1.0 years (1 to 5 years, 20 (32.3%) eyes; 6 to 10 years, 18 (29%) eyes, > 10 years, 24 (38.7%) eyes), 10.5±0.9 years (1 to 5 years, 18 (29%) eyes; 6 to 10 years, 18 (29%) eyes, > 10 years, 26 (42%) eyes), and 8.9±0.9 years (1 to 5 years, 8 (28.6 %) eyes; 6 to 10 years, 11 (39.3%) eyes, > 10 years, 9 (32.1%) eyes), respectively. Pre- and post-treatment tear samples were collected on filter paper strips and these strips were placed into an eppendorf tube. Tear activities of LDH, glucose-6-phosphate dehydrogenase (G6PDH), succine dehydrogenase (SDH), and acid phosphatase (AP), and tear contents of reduced and oxidized glutathione, and lipid peroxidation products (malondialdehyde and diene conjugates) were determined [29]. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software [12, 30]. Results and Discussion Table 1 presents the findings regarding tear activities of LDH and G6PDH in low or moderate myopes wearing either spectacles (control group) or SCL for up to 5 years and treated with different treatment regimens (SCL group).
After treatment, tear activities of LDH in myopes wearing SCL for up to 5 years and treated in subgroups 1, 2 and 3, decreased by 22.2% (almost to the norm), 23.8% (almost to the norm) and 4.9% respectively. There were no statistically significant differences in post-treatment tear activities of LDH between subgroups 1 and 2, but, relative to patients of subgroup 3, post-treatment tear activities of LDH among patients of subgroup 1 and among patients of subgroup 2 were statistically significantly lower. After treatment, tear activities of G6PDH in myopes wearing SCL for up to 5 years and treated in subgroups 1, 2 and 3, decreased by 20.8%, 19.2% and 2.9% (with no significant difference), respectively. There were no statistically significant differences in post-treatment tear activities of G6PDH between the subgroups. Table 2 presents the findings regarding tear activities of LDH and G6PDH in low or moderate myopes wearing either spectacles (control group) or SCL for 6 to 10 years and treated with different treatment regimens (SCL group).
After treatment, tear activities of LDH in myopes wearing SCL for 6 to 10 years and treated in subgroups 1, 2 and 3, decreased by 25%, 30.5% and 6.2% (with no significant difference), respectively. In addition, there were no statistically significant differences in post-treatment tear activities of the enzyme between subgroups 1 and 2. Moreover, post-treatment tear activities of the enzyme among patients of subgroup 1 and among those of subgroup 2 were not significantly different from the normal range. After treatment, tear activities of G6PDH in myopes wearing SCL for 6 to 10 years and treated in subgroups 1, 2 and 3, decreased by 22.2%, 23% and 5.7% (with no significant difference), respectively. In addition, post-treatment tear activities of the enzyme among patients of subgroup 1 and among those of subgroup 2 were not significantly different from the normal range. Table 3 presents the findings regarding tear activities of LDH and G6PDH in low or moderate myopes wearing either spectacles (control group) or SCL for > 10 years and treated with different treatment regimens (SCL group). After treatment, tear activities of LDH in myopes wearing SCL for >10 years and treated in subgroups 1, 2 and 3, decreased by 31.4%, 34.8% and 5.8% (with no significant difference), respectively. In addition, there were no statistically significant differences in post-treatment tear activities of the enzyme between subgroups 1 and 2, but, relative to patients of subgroup 3, post-treatment tear activities of the enzyme among patients of subgroup 1 and among patients of subgroup 2 were statistically significantly lower.
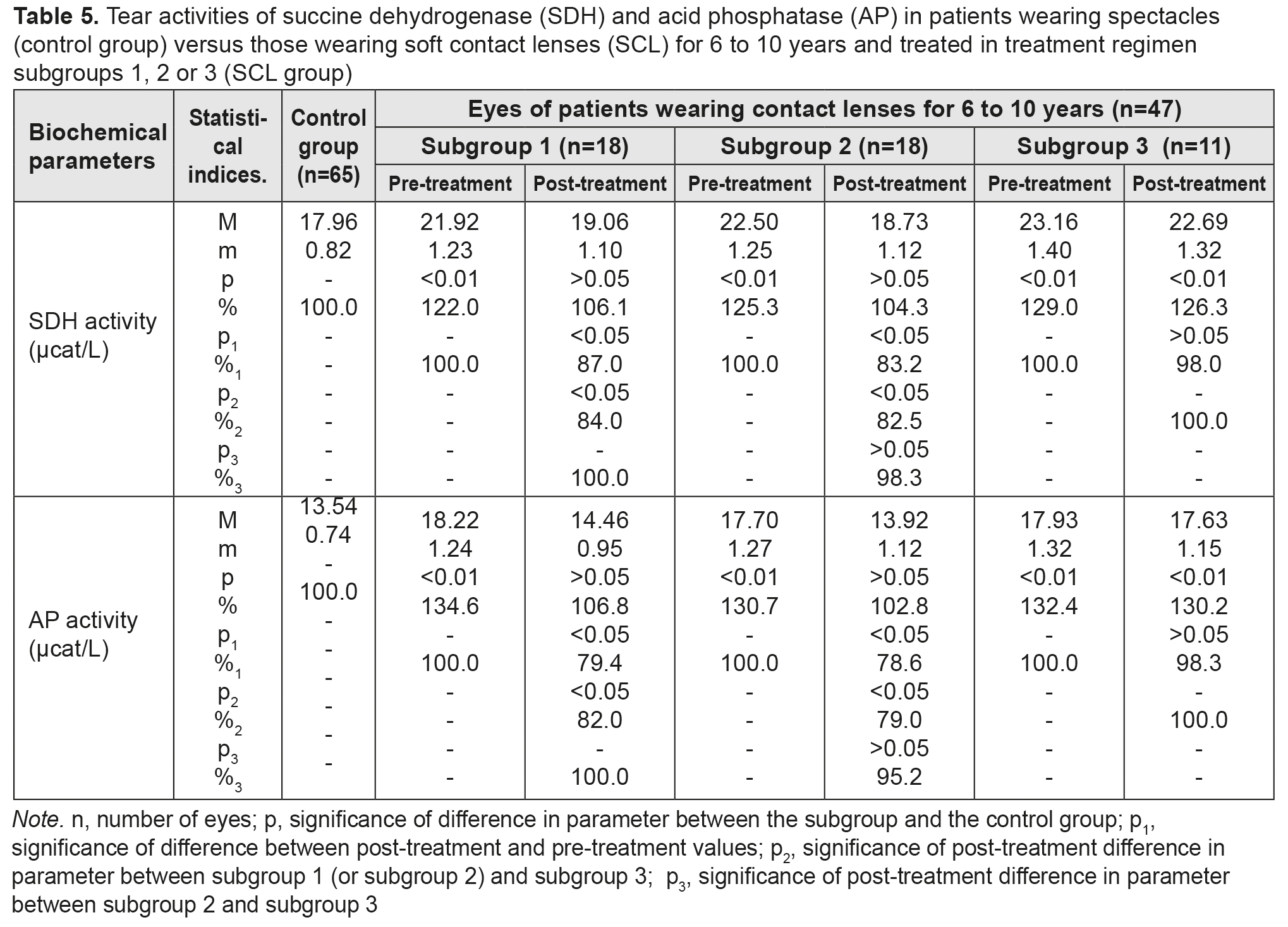
After treatment, tear activities of G6PDH in myopes wearing SCL for >10 years and treated in subgroups 1, 2 and 3, decreased by 27.9%, 33.1% and 7.5% (with no significant difference), respectively. In addition, there were no statistically significant differences in post-treatment tear activities of the enzyme between subgroups 1 and 2. Moreover, post-treatment tear activities of the enzyme among patients of subgroup 1 and among those of subgroup 2 were not significantly different from the normal range. Tear activities of LDH and G6PDH reflect a degree of epithelial destruction, since these enzymes are located in the cytoplasm of epithelial cells. The finding of elevated tear activities of these two enzymes provided the evidence for contact lens-induced intensive destruction of corneal cells and subcellular structures. The data provided in Tables 1 to 3 demonstrate that there is a direct relationship between duration of SCL wear and activities of these enzymes in the tear fluid, which suggests increased destruction of ocular surface cells. In addition, thiotriazolin was found to improve these parameters irrespective of its route of administration. Although the treatment regimen including electrophoresis with thiotriazolin was found to decrease tear activities of LDH and G6PDH to a somewhat greater extent than other regimens, this improvement was not statistically significant. Table 4 presents the findings regarding tear activities of SDH and AP in low or moderate myopes wearing either spectacles (control group) or SCL for up to 5 years and treated with different treatment regimens (SCL group). At baseline, tear SDH and AP levels were found to be elevated in SCL wearers, and to depend on duration of SCL wear (Table 4). After treatment, tear activities of SDH in myopes wearing SCL for < 5 years and treated in subgroups 1, 2 and 3, decreased by 10.5%, 12.1% and 3.2% (with no significant difference), respectively. There were no statistically significant differences in post-treatment tear activities of the enzyme between subgroups 1, 2 and 3.
After treatment, tear activities of AP in myopes wearing SCL for <5 years and treated in subgroups 1 and 2, decreased by 10.5% and 15.1%, respectively, but subgroup 3 showed no decrease in this parameter. Post-treatment tear activities of AP among patients of subgroups 1 and 2 were not statistically significantly different from those among patients of subgroup 3. Table 5 presents the findings regarding tear activities of SDH and AP in low or moderate myopes wearing either spectacles (control group) or SCL for 6 to 10 years and treated with different treatment regimens (SCL group). After treatment, in myopes wearing SCL for 6 to 10 years and treated in subgroups 1, 2 and 3, tear activities of the former enzyme decreased by 13%, 16.8% and 2% (with no significant difference), respectively, and those of the latter anzyme decreased by 20.6%, 21.6% and 1.7% (with no significant difference), respectively.
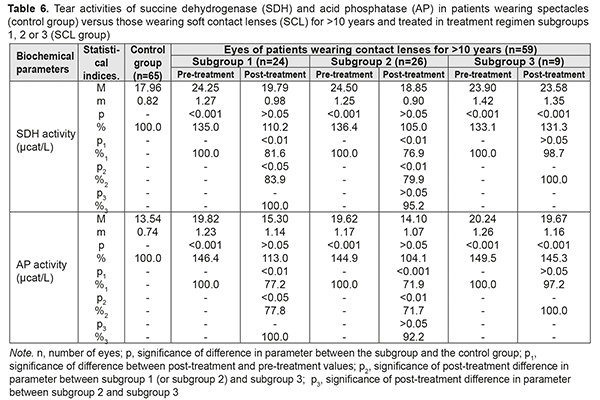
Table 6 presents the findings regarding tear activities of SDH and AP in low or moderate myopes wearing either spectacles (control group) or SCL for >10 years and treated with different treatment regimens (SCL group). After treatment, in myopes wearing SCL for >10 years and treated in subgroups 1, 2 and 3, tear activities of the former enzyme decreased by 18.4%, decreased by 23.1%, and did not decrease, respectively, and those of the latter anzyme decreased by 22.8%, 28.1% and 2.8% (with no significant difference), respectively. In addition, after treatment, for any duration of SCL wear, (a) there were no statistically significant differences in tear activities of SDH and AP between subgroups 1 and 2, and (b) these activities among patients of both subgroups were close to the normal range.
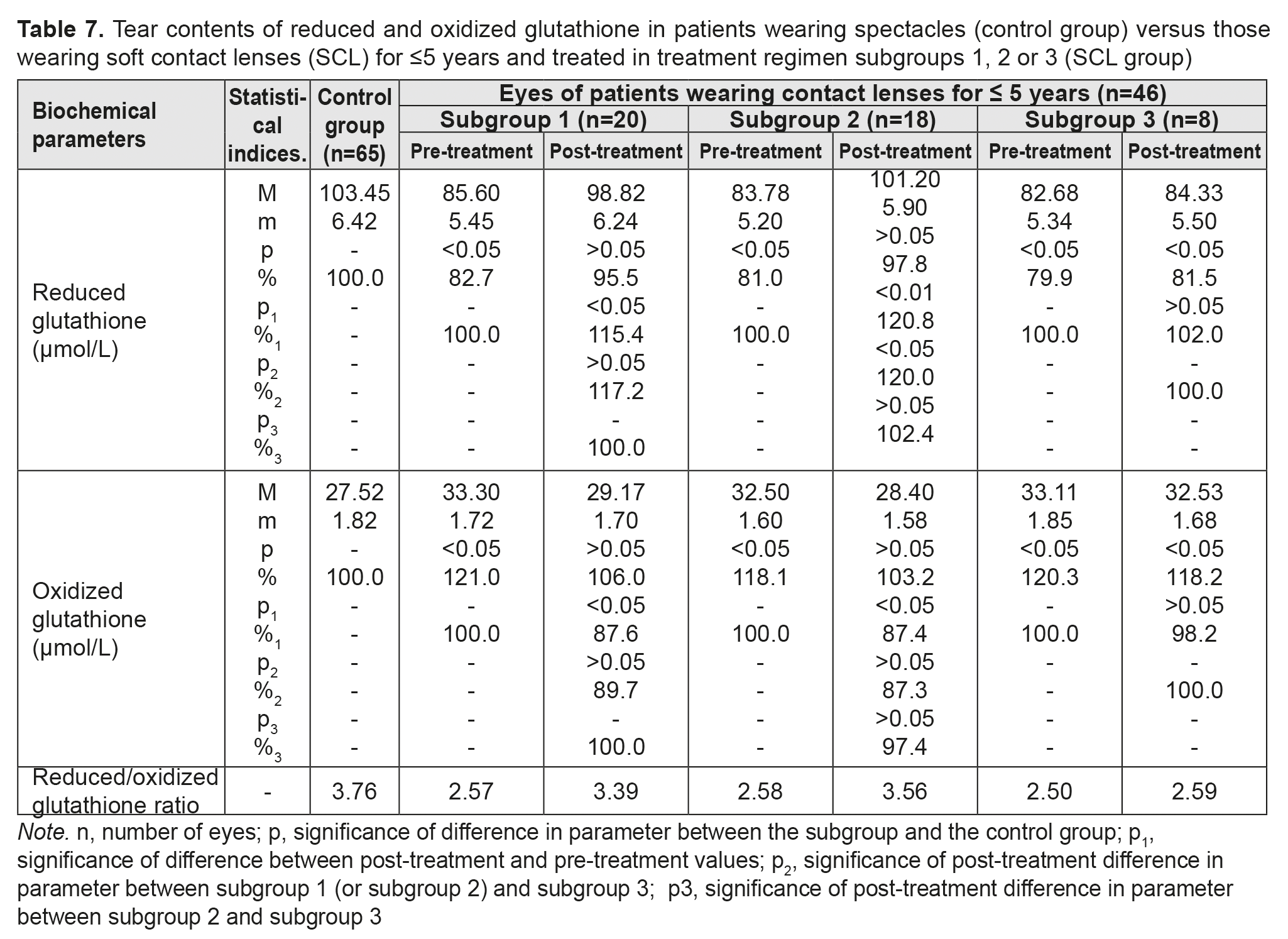
Table 7 presents the findings regarding tear contents of reduced and oxidized glutathione in low or moderate myopes wearing either spectacles (control group) or SCL for <5 years and treated with different treatment regimens (SCL group). We found that, in any subgroup of the SCL group, pre-treatment tear contents of reduced and oxidized glutathione were diminished and elevated, respectively, indicating low antioxidant capacity in the anterior eye. Thiol system imbalance results in a reduced defensive-and-adaptive capacity of the cornea and other anterior eye structures in individuals wearing contact lenses for a long time.
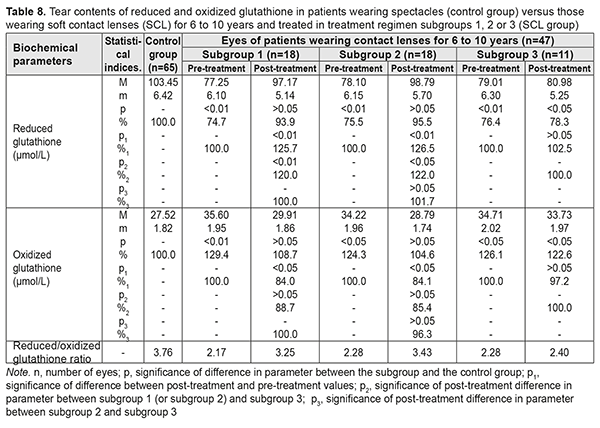
After treatment, in myopes wearing SCL for <5 years and treated in subgroups 1, 2 and 3, tear contents of reduced glutathione increased by 15.4%, increased by 20.8%, and did not change significantly, respectively, whereas tear contents of oxidized glutathione decreased by 15.4%, decreased by 20.8%, and did not change significantly, respectively. Table 8 presents the findings regarding tear contents of reduced and oxidized glutathione in low or moderate myopes wearing either spectacles (control group) or SCL for 6 to 10 years and treated with different treatment regimens (SCL group). After treatment, in myopes wearing SCL for 6 to 10 years and treated in subgroups 1, 2 and 3, tear contents of reduced glutathione increased by 25.7%, increased by 26.5%, and did not change significantly, respectively, whereas tear contents of oxidized glutathione decreased by 16.0%, decreased by 15.9%, and did not change significantly, respectively.
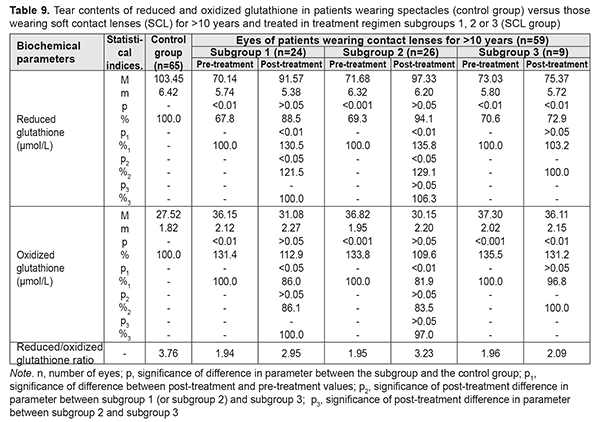
Table 9 presents the findings regarding tear contents of reduced and oxidized glutathione in low or moderate myopes wearing either spectacles (control group) or SCL for >10 years and treated with different treatment regimens (SCL group). After treatment, in myopes wearing SCL for >10 years and treated in subgroups 1, 2 and 3, tear contents of reduced glutathione increased by 30.5%, 35.8%, and 3.2% (an increase in subgroup 3 was not statistically significant), respectively, whereas tear contents of oxidized glutathione decreased by 14.0%, decreased by 18.1%, and did not change significantly, respectively. With regard to post-treatment tear contents of reduced and oxidized glutathione, there were no statistically significant differences between subgroups 1 and 2 for any CL-wearing period, and parameters among patients of both these subgroups were close to those of patients of the spectacle group.
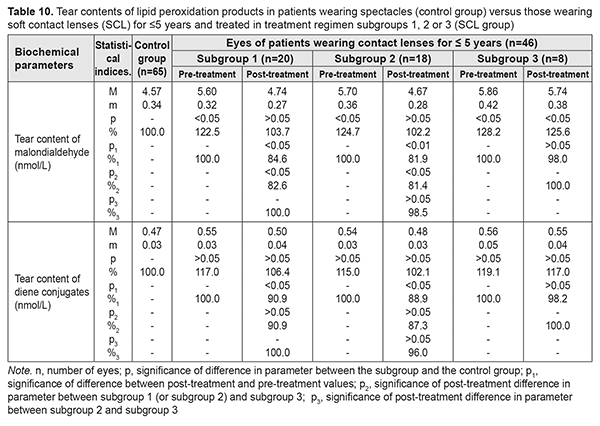
Table 10 presents the findings regarding tear contents of LPO products in low or moderate myopes wearing either spectacles (control group) or SCL for <5 years and treated with different treatment regimens (SCL group). After treatment, in myopes wearing SCL for <5 years and treated in subgroups 1 and 2, tear content of malondialdehyde (MDA) decreased by 15.4% and 18.1%, respectively, and that of diene conjugates decreased by 9.1% and 11.1%, respectively, while both these parameters did not change significantly in subgroup 3. With regard to post-treatment tear contents of MDA and diene conjugates, there were no statistically significant differences between subgroups 1 and 2.
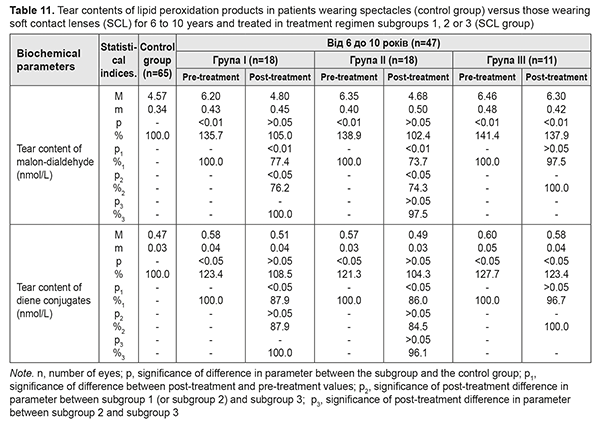
Table 11 presents the findings regarding tear contents of LPO products in low or moderate myopes wearing either spectacles (control group) or SCL for 6 to 10 years and treated with different treatment regimens (SCL group). After treatment, in myopes wearing SCL for 6 to 10 years and treated in subgroups 1 and 2, tear content of MDA decreased by 22.6% and 26.3%, respectively, and that of diene conjugates decreased by 12.1% and 11.4%, respectively. In addition, these parameters decreased by 2-3% in subgroup 3, although not statistically significantly.
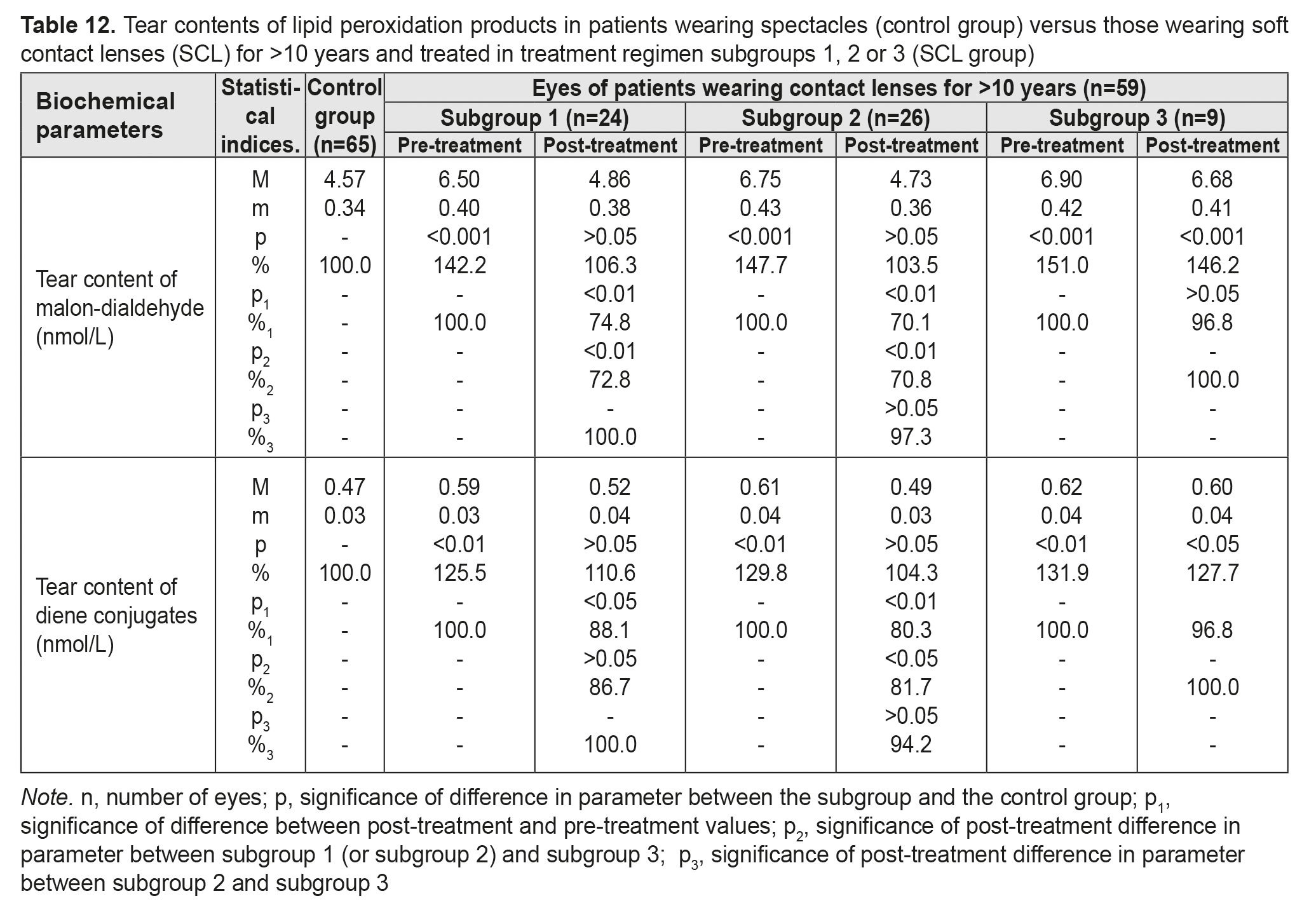
Table 12 presents the findings regarding tear contents of LPO products in low or moderate myopes wearing either spectacles (control group) or SCL for >10 years and treated with different treatment regimens (SCL group). After treatment, in myopes wearing SCL for >10 years and treated in subgroups 1 and 2, tear content of MDA decreased by 25.2% and 29.9%, respectively, and that of diene conjugates decreased by 11.9% and 19.7%, respectively. In addition, these parameters decreased by 3.2% in subgroup 3, although not statistically significantly.
Conclusion Activation of free radical processes and reduced antioxidative reserves lead to disruption of cellular and subcellular membranes of ocular surface epithelial cells (including, in particular, corneal epithelial cells) and play a crucial role in the pathogenesis of neurotrophic keratoconjunctivitis in individuals wearing soft contact lenses for years. In patients wearing SCL for different time periods, our one-month multi-component therapy regimen 1 including topical and oral morpholinium-methyl-triazolyl-thioacetate (thiotriazolin) resulted in decreases in tear LDH, G6PDH, SDH and AP levels by 22-31%, 19-28%, 10-18%, and 10-23%, respectively. In addition, it resulted in increases in tear contents of reduced and oxidized glutathione by 15-30% and 12-14%, respectively, and decreases in tear contents of secondary LPO product (malondialdehyde) and primary LPO products (diene conjugates) by 15-25% and 9-12%, respectively. Our two-week multi-component therapy (regimen 2) including topical thiotriazolin and electrophoresis using thiotriazolin decreased tear LDH, G6PDH, SDH and AP levels by 24-35%, 19-33%, 12-23%, and 15-28%, respectively. In addition, it increased tear contents of reduced and oxidized glutathione by 21-36% and 12-18%, respectively, and decreased tear contents of malondialdehyde and diene conjugates by 18-30% and 11-20%, respectively. No statistically significant changes in the investigated parameters were found in myopes wearing SCL for different time periods and treated with topical antiseptic and hyaluronic acid artificial tears only, without adjunctive thiotriazolin (regimen 3). No statistically significant differences in treatment results between subgroups 1 and 2 were found, although in subgroup 2, the results were somewhat better, and the treatment course was shorter. The use of thiotriazolin in the treatment of neurotrophic keratoconjunctivitis in individuals wearing SCL for years resulted in a statistically significant decrease in membrane lipid peroxidation activity due to restoration of prooxidative-antioxidative status, thus leading to stabilization of cell membranes and subcellular structures (organelles) of the ocular surface, in particular the cornea. Therefore, it was proved that thiotriazolin protects the ocular surface from oxidative stress. All of these point to the efficacy of pathogenetic treatment with thiotriazolin for neurotrophic keratoconjunctivitis in wearers of SCL for long periods, and to the potential for prevention of severe complications of vision correction with SCL.
References 1.Asmolova AA. [Maxillary postimplantation syndrome: abnormal variant anatomy of the paranasal sinuses]. Luchevaia diagnostika, luchevaia terapiia. 2015; 3–4:47–53. Russian. 2.Asmolova AA. [Maxillary postimplantation syndrome: clinical observations]. Luchevaia diagnostika, luchevaia terapiia. 2018; 1:78–81. Russian. 3.Asmolova AA. [Maxillary postimplantation syndrome: status of carotid arteries]. Luchevaia diagnostika, luchevaia terapiia. 2016; 1:22–7. Russian. 4.Asmolova AA. [Status of oral fluid in maxillary postimplantation syndrome]. Innovatsii v stomatologii. 2017; 1:11–4. Russian. 5.Аsmolova AА. Dental implants can generate maxillary postimplantation syndrome. Arta Medica. 1(54):28–30. 6.Lapach SN, Chubenko AV, Babych PN. [Statistical methods in biomedical research using Excel]. 2nd ed. Kyiv: Morion; 2001. Ukrainian. 7.Sheremet NL. [Diagnostics of optical neuropathies of different origin]. Dissertation for the degree of Doctor of Medical Science. Moscow: Research Institute for Eye Disease; 2015. Russian. 8.Chang MY, Pineles SL. Optic disc drusen in children. Surv Ophthalmol. 2016 Nov - Dec;61(6):745-58. 9.Fotzsch R. Problems in the etiology, pathogenesis and clinical significance of drusen (hyalin bodies) of the optic disk. Psychiatr Neurol Med Psychol (Leipz). 1970 Jun;22(6):223-7.
|