J.ophthalmol.(Ukraine).2018;6:35-39.
|
http://doi.org/10.31288/oftalmolzh201863539 Received: 10 September 2018; Published on-line: 31 December 2018 Characteristics of crystallographic changes in tear in different stages of diabetic retinopathy I.M. Bezkorovayna, Dr Sc (Med), Prof.; D.O. Nakonechnyi; A.O. Bezkorovayna Ukrainian Medical Stomatological Academy; Poltava (Ukraine) E-mail: nakone4nyiden@gmail.com TO CITE THIS ARTICLE: Bezkorovayna I.M., Nakonechnyi D.O., Bezkorovayna A.O. Characteristics of crystallographic changes in tear in different stages of diabetic retinopathy. J.ophthalmol.(Ukraine).2018;6:35-39. http://doi.org/10.31288/oftalmolzh201863539
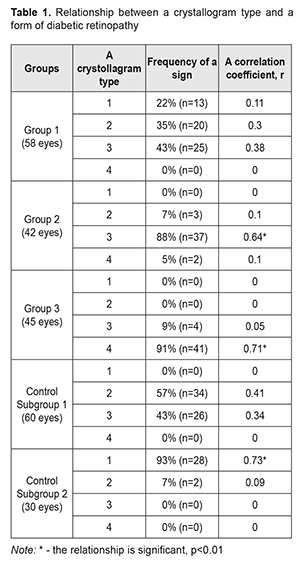
Introduction. Studying the specific crystallographic signs of tear in the development of diabetic retinopathy is of an extraordinary interest in clinical practice. Aim. To study the changes in the crystollografic signs of tear depending on a stage of diabetic retinopathy. Materials and methods. Under our supervision, there were 84 patients (145 eyes) with diabetes mellitus. Patients were divided into three groups according to the classification of diabetic retinopathy by N.V. Pasechnikova and V.O. Naumenko, adopted by the XII-th Ukrainian Congress of Ophthalmologists. Group 1 consisted of 36 patients (58 eyes) with non-proliferative diabetic retinopathy (NPDRP); Group 2 consisted of 25 patients (42 eyes) with pre-proliferative diabetic retinopathy (PPDRP); Group 3 consisted of 23 patients (45 eyes) with proliferative diabetic retinopathy (PDRP). Results. A vast intermediate area and single point inclusions in the amorphous and intermediate areas of the tear facie was noted in 81% of the NPDRP patients (р<0.01); the pathologic crystallization in the amorphous area of the tear facie and a prevalence of large-size crystals and vast intermediate areas was in 74% of the PPDRP patients (р<0.01); and the increased density of crystal arrangement and single brown crystals were in 93% of the PDRP patients (р < 0.01). Conclusions. A relationship was defined between the changes in the tear crystallography type in the patients with PPDRP and PDRP, who had a crystollogram of type 3 and type 4, respectively, (p <0.01). The additional crystallographic sings of tear, the changes in which correspond to different stages of DRP, were also defined. Keywords: diabetic retinopathy, native crystallography of tears, specific crystallographic sings Introduction Non-invasive techniques for assessing the qualitative composition of tear, which are based on studying biochemical and immunological properties in different forms of diabetic retinopathy (DRP), are known worldwide [2, 9]; they, however, have not come into the common use in Ukraine because of the need for expensive equipment and reagents. Ukraine’s scientists have been studied native crystallography of tear, an alternative method which is quite simple to use and which is an indicator of misbalance in tear components [3, 4, 5, 7]. Late diagnostics of DRP makes it difficult to perform adequate pathogenically-oriented correction of the detected disorders and it is one of the major causes of patients' incapacitation [1, 6, 8, 10, 11]. In the literature, to the best of our knowledge, there is no data on the specific chrystollographic changes in tear in different DRP stages, which gives no chance to use it widely in the clinical practice. Thus, the studies on these issues and the further implementation of their findings in the clinical practice are of a certain interest. Purpose. To study the changes in the crystollografic signs of tear depending on a stage of diabetic retinopathy. Material and Methods We followed up eighty-four patients (145 eyes) with diabetes mellitus, who were under medical treatment at Endocrinology Department of Poltava Region Clinical Hospital named after M.V. Sklifosovsky and Regional Primary Health Care Centre. The patients aged 63±0.04 with a range from 56 to 80 years. There were 51 (61%) women and 33 (39%) men. All patients were under the care of an endocrinologist and cardiologist and received glucose lowering and antihypertensive therapy. In addition, all patients consulted an ophthalmologist. All patients in Study groups were prescribed basic glucose lowering therapy; besides, 29 patients (35%) were additionally prescribed fenofibrate, 145 mg, once a day. 26 patients (31%) had had laser photocoagulation of the retina in history; the rest of the patients had not been performed laser treatment due to a number of objective and subjective reasons. The followed-up patients were divided into three groups based on the DRP classification by Pasyechnikova NV and Naumenko VA which had been accepted by The XII Ukrainian Congress of Ophthalmologists. Group 1 consisted of 36 patients (58 eyes) with non-proliferative diabetic retinopathy (NPDRP); Group 2 consisted of 25 patients (42 eyes) with pre-proliferative diabetic retinopathy (PPDRP); Group 3 consisted of 23 patients (45 eyes) with proliferative diabetic retinopathy (PDRP). The best-corrected ETDRS visual acuity was 0.71±0.02, 0.3±0.03, and 0.08±0.01 in Groups 1, 2, and 3, respectively. In the ocular fundus, there were revealed: in Group 1, single microaneurysms in 58 eyes (100%), retinal hemorrhages in 19 eyes (33%), and cotton wool spots in 11 eyes (19%); in Group 2, microaneurysms and hemorrhages in the four quadrants in 34 eyes (81%), venous beading in 19 eyes (45%), intraretinal microvascular abnormalities in 18 eyes (43%); in Group 3, new vessels on the disc in 45 eyes (100%), pre retinal hemorrhages in 20 eyes (44%), tractional retinal detachment in 1 eye (2%), vitreous hemorrhage in 3 eyes (7%). Control group consisted of two subgroups without diabetes mellitus: Subgroup 1, 30 patients (60 eyes) with dry eye syndrome; and Subgroup 2, 15 patients (30 eyes), ophthalmologically healthy persons. Visual fields corresponded to the severity of the fundus alterations. To determine the severity of dry eye syndrome (DYS) against the background of diabetes mellitus (DM), we measured: total tear secretion (Schirmer's test I); tear film stability (the Norn’s test); and lid-parallel conjunctival folds (LIPCOF) in the inferior lateral quadrant. The tests’ findings were evaluated as follows: age-appropriate normal values, the Schirmer's test >10 mm/5 min, the Norn’s test >10s, LIPCOF=0; mild DES, the Schirmer's test ≤10 mm/5 min, the Norn’s test ≤10s, LIPCOF=1; moderate DES, the Schirmer's test ≤5mm/5 min, the Norn’s test ≤10s, LIPCOF=2; severe DES, the Schirmer's test ≤2mm/5 min, the Norn’s test ≤2s, LIPCOF=3; The tear was collected for crystallography from the inferior fornix conjunctiva using sterile graduated pipettes; the bio-substrate was placed into the Eppendorf tubes for no more than 2 hours. Afterwards, a drop of the bio-substrate was drawn with an insulin syringe on a clean microscope slide placed horizontally. The specimen was dried out at a temperature of 20-25ºС and relative humidity of 65-70% for 24 hours. Indices were fixed twice within 24 hours using a thermo-hygrometer TFA 45204250. Crystallograms were studied after the time specified for obtaining a thin dry film (facies) had been expired. A microscopic crystallography examination was performed using a light microscope at a 30, 100, 200, and 800 × magnifications and a photomicrographic attachment was used to take photographs. In cases of a lack of tears and changes in tear viscosity, mainly in the patients with proliferative DRP, the lacrimal points were blocked by obturators. In such cases, the time of collecting the specimen in one eye was up to 10 minutes. The examination findings referred to how a picture of the crystallized tear coincided with one of four types as follows [2]: Type 1: the crystal structures are evenly distributed throughout the field of view without gaps between the fern patterns. Single fern patterns of a large size are tightly branched out. Type 2: a crystallographic picture is similar to Type 1 crystallization; however, single fern patterns are of a smaller size and branched out with a lower frequency compared to Type 1. Empty spaces appear between the fern patterns. Type 3: a crystallographic picture is normal only in particular areas. Standing-alone fern patterns are of a small size with construction defects and with rare or completely absent branches. There are vast empty spaces with unordered mucin inclusions. Type 4: no phenomenon of fern-like patterns. In the field of view, there are mucus masses and threads, which are denatured mucin mixed degeneratively-changed epithelial cells of the ocular surface. In addition, we analyzed the crystallographic signs which were beyond the description of the types above. All these signs were scored according to the presence or the intensity (0, 1, or 2 points). Afterwards, these signs were studied in regard of their correlation with stages of DRP. Statistical processing of the data was performed using a software package (STATISTICA 6.0, StatSoft. Inc., the USA), and a descriptive statistics software package (EXEL). Results and Discussion In Group 1, no correlation was detected between a crystollogram type and NPDRP. In Group 2, there was a direct correlation noted between PPDRP and a Type 3 crystallogram; in Group 3, a direct correlation was noted between PDRP and a Type 4 crystallogram. In Control Group, there was a correlation between Subgroup 2 and a Type 1 crystallogram; no correlation was noted in Subgroup 2 (Table 1).
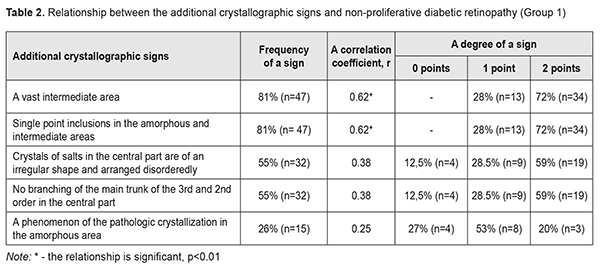
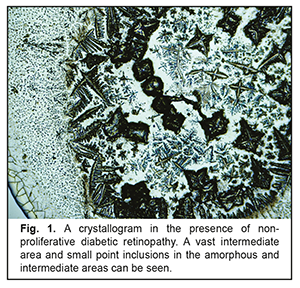
When analyzing the crystallograms, the additional crystallographic sings were noted in the study groups. Thus, in Group 1 with NPDRP, a direct relationship was noted between the presence of a vast intermediate area and small point inclusions in the amorphous and intermediate areas. Their combination with other additional crystallographic signs, which were observed in 31 eyes (53%), was not significant p<0.05 (Table 2, Fig. 1).
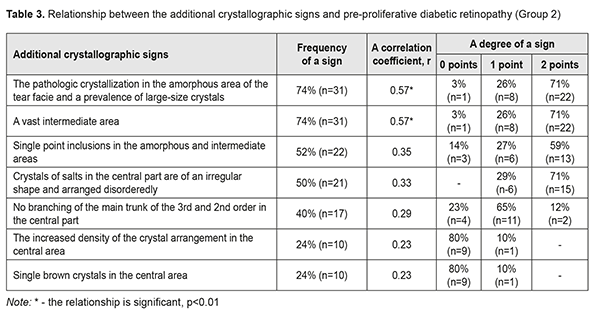
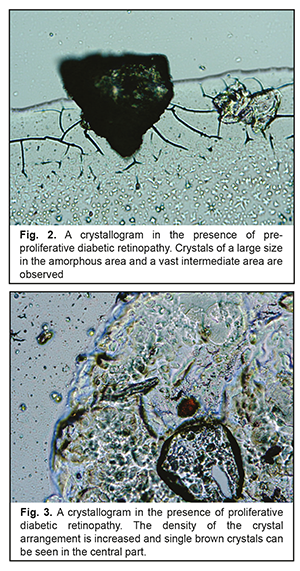
In Group 2 with PPDRP, a direct relationship was noted between the pathologic crystallization in the amorphous area of the tear facie and a prevalence of large-size crystals and a vast intermediate area. Their combination with other additional crystallographic signs, which were observed in 24 eyes (57%), was not significant p<0.05 (Table 3, Fig. 2).
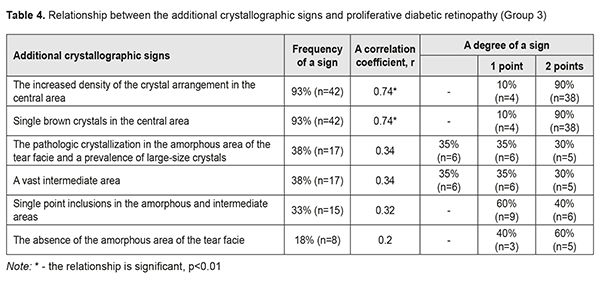
In Group 3 with PDRP, a direct relationship was noted between the increased density of crystal arrangement and single brown crystals in the central part of the tear facie. Their combination with other additional crystallographic signs, which were observed in 18 eyes (40%), was not significant p<0.05 (Table 4, Fig. 3).
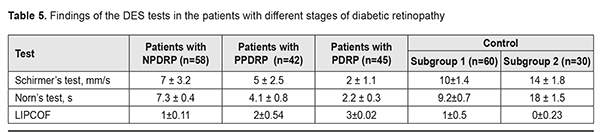
Findings on the severity of DES (The Schirmer’s test, LIPCOF, and the Norm’s test) are given in Table 5. In our studies, the DES severity had a direct correlation with the changes in the crystallograms. Thus, the patients with NPDRP had mild DES in 58% (r=0.61, p<0.01), and normal ranges in 42% (r=0.34, p<0.05). In this group, the relationship between a crystallography type and NPDRP was not significant; however, there was a relationship with the additional crystallographic signs: a vast intermediate area and single point inclusions in the amorphous and intermediate areas of the tear facie. The patients with PPDRDP had moderate and mild DES in 74% (r=0.72, p<0.01) and 26% (r=0.17, p>0.05), respectively. In this group, a type 3 crystallogram was noted with the crystallographic signs including the pathologic crystallization in the amorphous area of the tear facie and a prevalence of large-size crystals and a vast intermediate area. The patients with PDRP had severe DES in 100% (r=0.79, p<0.01) and a type 4 crystallogram with the additional crystallographic signs including the increased density of crystal arrangement and single brown crystals in the central part of the tear facie.
Thus, a vast intermediate area and single point inclusions in the amorphous and intermediate areas of the tear facie was noted in 81% of the NPDRP patients (р<0.01); the pathologic crystallization in the amorphous area of the tear facie and a prevalence of large-size crystals and vast intermediate areas was in 74% of the PPDRP patients (р<0.01); and the increased density of crystal arrangement and single brown crystals was in 93% of the PDRP patients (р < 0.01). To conclude, firstly, a significant relationship was defined between a tear crystallography type and the patients with PPDRP and PDRP, who had a crystollogram of type 3 and type 4, respectively. Secondly, a relationship was defined between the occurence of additional crystallographic signs of the tear and NPDRP (a vast intermediate area and single point inclusions in the amorphous and intermediate areas of the tear facie). Thirdly, a relationship was defined between the occurence of the additional crystallographic signs of the tear and PPDRP (the pathologic crystallization in the amorphous area of the tear facie and a prevalence of large-size crystals and vast intermediate areas). Finally, a relationship was defined between the occurence of the additional crystallographic signs of the tear and PDRP (the increased density of crystal arrangement and single brown crystals).
References 1.Bezkorovayna IM, Voskresenska LK, Riadnova VV, Shatkun AA. [Clinical features of changes in the retina depending on a type of diabetes mellitus]. Vrachebnoie delo. 2016;7–8(1140):104-8. Ukrainian. 2.Brzheskii VV, Somov EE. [Tear fluid as a biological material for diagnostic testing]. [Current issues of pediatric ophthalmology: Proceedings]. SPb:1995;28-31. Russian. 3.Zavgorodnyaya, NG, Isakova OA. [Early diagnosis of the syndrome of "dry eye" to assess the qualitative composition of tears]. Oftalmol Zh. 2005;5:18-20. Russian. 4.Zavgorodnyaya NG, Brizhan AA. [Cytologic status changes of the conjunctiva and tears qualitative composition in patients with «dry eye» syndrome after instillation of modern topical fl uoroquinolones]. Zaporozhskii med. zhurnal. 2014;3(84):52-8. Russian. 5.Moshetova LK, Volkov OA. [A contemporary view of tear fluid, its role in diagnostics]. Klinicheskaia oftalmologiia. 2004;5(4):138-9. Russian. 6.Pasechnikova NV, Naumenko VA, Zborovskaya AV, Kushnir NN, Yakovenko TA. [State of hematoretinal barrier in diabetic retinopathy according to the data of fluorometry]. Oftalmol Zh. 2008;5:4-7. Russian. 7.Shabalin VN, Shatokhina SN et al. [Morphology of liquid fluids of the eye]. M.: Meditsina; 2004. 243 p. Russian. 8.International Diabetes Federation. Available from: http://www.idf.org/diabetesatlas. 9.Grus FH, Augustin AJ, Evangelou NG, Toth-Sagi K. Analysis of tear-protein patterns as a diagnostic tool for the detection of dry-eyes in diabetic and non-diabetic dry-eye patients. Eur J Ophthalmol. 1998;8(2):90-7. 10.Richard G. Fluorescein and ICG angiography. Thieme Medical Publishers, Inc., New York; 1998. 369 p. 11.Walker J, Rykov SA, Suk SA, Saksonov SG. [Diabetic retinopathy: in a simple way about a complex thing].Kyiv;2013. 320 p. Russian.
|