J.ophthalmol.(Ukraine).2018;6:30-34.
|
http://doi.org/10.31288/oftalmolzh201863034 Received: 27 August 2018; Published on-line: 31 December 2018 Intraocular temperature changes during vitrectomy procedure R.E. Nazaretyan1, O.S. Zadorozhnyy1, Cand Sc (Med), M.M. Umanets1, Dr Sc (Med), V.A. Naumenko1, Dr Sc (Med), Prof., N.V. Pasyechnikova, Assoc. Member of the NAMS of Ukraine, Dr Sc (Med), Prof., V.V. Shafranskii2, Dr Sc (Med), Assoc. Prof. 1 Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine; Odesa (Ukraine) 2 Bohomolets National Medical University; Kyiv (Ukraine) E-mail: laserfilatova@gmail.com TO CITE THIS ARTICLE: Nazaretyan RE, Zadorozhnyy OS, Umanets MM, Naumenko VA, Pasyechnikova NV, Shafranskii VV. Intraocular temperature changes during vitrectomy procedure. J.ophthalmol.(Ukraine).2018;6:30-34.http://doi.org/10.31288/oftalmolzh201863034
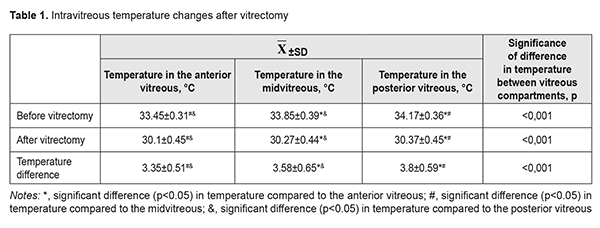
Background: Monitoring of treatment parameters is of utmost importance in current medicine. The use of intraocular media and irrigation fluid temperature monitoring during vitreoretinal surgery is, however, not common in current clinical practice. Purpose: To investigate intraocular temperature changes at various time points of vitreoretinal surgery. Materials and Methods: Twenty patients (20 eyes) who underwent vitrectomy with room-temperature (24.2±0.52 ˚С) irrigation solutions at an ambient temperature of 24.4±0.51 °С were under observation. Temperatures in the anterior, mid- and posterior vitreous were recorded before and immediately after vitrectomy and after performance of additional surgical manipulations. Results: The presence of a transvitreous temperature gradient from the anterior toward the posterior vitreous of the human eye was confirmed. At baseline, the highest temperature (34.17±0.36 °С) was recorded in the posterior vitreous. There were significant decreases in temperatures in vitreous compartments immediately after vitrectomy, with the lowest temperature of 30.1±0.45 °С recorded in the anterior vitreous, and the greatest temperature decrease (3.8±0.59 ˚С) compared to baseline, in the preretinal posterior vitreous. There were increases in temperatures in vitreous compartments after additional surgical manipulations and, with an increase in the duration of these manipulations, temperature in the vitreous increased at an average rate of 0.18°C per minute. Conclusion: Vitreoretinal surgery is commonly performed under conditions of artificially induced local hypothermia, warranting intraoperative monitoring of intraocular and irrigation fluid temperatures. Keywords: vitrectomy, intraocular temperature, human eye Introduction Although pars plana vitrectomy has a relatively long history, there are still some issues to be solved in this field. Thus, phototoxic damage and even thermal damage to the retina during vitrectomy have been reported. This is due to the fact that, during vitrectomy procedures, the media provide no protection of the retina from the light of the endoilluminator [1]. In addition, toxic effects of vitrectomy dyes on the retinal neuroepithelium [2] and mechanical damage to the inner retina from air infusion during vitrectomy have been described [3]. Moreover, intraocular pressure rise and systemic arterial pressure decrease during vitreoretinal surgery can cause a dangerous decrease in perfusion pressure resulting in intraoperative ischemic retinal and optic nerve damage [4]. It remains poorly understood what should be the temperature of the irrigation solution for intraocular surgery and how long it is reasonable to use irrigation solutions during vitrectomy [5]. As early as 1986, Rinkoff et al [6] demonstrated in rabbits that low-temperature solutions can be used in vitreoretinal surgery to reduce retinal damage from endoilluminator light. In the study by Tamai and colleagues [7], ischemia in the rabbit eye was induced by increasing intraocular pressure during vitrectomy; the least structural changes in the retina were noted in the rabbits which underwent closed vitrectomy with their vitreous cavities irrigated with solution of low temperature. Romano and colleagues [8] found that the variations in temperature during vitreoretinal surgery are clinically significant, as the rheology of tamponades can be better manipulated by modulating intraocular pressure and temperature. The use of intraocular temperature monitoring during vitreoretinal surgery conducted under hypothermic conditions will make it possible to track and regulate changes in eye temperature. This, in its turn, will contribute to a more rational use of beneficial thermal effects on the eye, with improvement in intra- and post-operative complication rate and treatment outcomes. The purpose of the study was to investigate intraocular temperature changes at various time points of vitreoretinal surgery. Materials and Methods This was an open pilot study. The study protocol was approved by a local Bioethics Committee of the Filatov Institute. Written informed consent was obtained from all individual participants included in the study. Twenty patients (20 eyes, including 6 with diabetic retinopathy, 4 with vitreous hemorrhage, 8 with rhegmatogenous retinal detachment, and 2 with macular hole) were under observation. Patient age ranged from 37 to 65 years. 23-G pars plana vitrectomy was performed using the Alcon Constellation vitrectomy machine (Alcon Laboratories, Inc., Fort Worth, TX, USA). The duration of vitrectomy was calculated as the time the vitrectomy was initiated to the time of the end of the core vitrectomy phase. Technique The surgical site was prepared with antiseptic solution and epibulbar and subtenon anesthesia was administered. Thereafter, a standard three-port vitrectomy was performed with cutting rates of 3500-7000 cuts/min, aspiration pressure of 300-650 mm Hg, and irrigation pressure of 25 mm Hg. Room-temperature BSS PLUS® (Alcon Laboratories, Inc., Fort Worth, USA) was used as an intraocular irrigating solution. The temperature of the irrigating solution delivered into the eye was monitored and regulated during surgery. In addition, ambient temperature in the operating room, and patient’s body temperature, blood pressure, heart rate and blood oxygen saturation were recorded. Temperatures in the anterior, mid- and posterior vitreous were measured before vitrectomy, immediately after surgery, and after performance of all additional manipulations such as endolaser retinal photocoagulation, removal of the internal limiting membrane (ILM), and retinal flattening with perfluorodecalin. A thermoelectric device [9, 10] developed by the Institute of Thermoelectricity of the NAS of Ukraine and MES of Ukraine, and the Filatov Institute was used for measuring temperatures of various ocular structures, irrigating solution, and operating room. Statistics Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) and MedCalc 18.10 (MedCalc Software Inc, Broekstraat, Belgium) software. Means and standard deviations (SD) were calculated. The level of significance p ≤ 0.05 was assumed. Repeated measures ANOVA with Bonferroni correction were used for pairwise comparison (for normally distributed data), whereas Friedman test for related samples and Conover tests were used for pairwise comparison (for non-normally distributed data) of temperatures in different vitreous compartments. Logistic regression models were used to examine the relationships of baseline vitreous temperature with patient’s diagnosis, body temperature, blood pressure, heart rate and blood oxygen saturation. Regression analysis was used to identify the relationship of a change (Δ) in midvitreous temperature after vitrectomy with duration of additional surgical manipulation. Results At baseline (time point 1), mean operating room temperature was 24.4±0.51 ˚С, mean irrigation solution temperature was 24.2±0.52 ˚С, and mean patient body temperature was 36.58±0.08 ˚С. In addition, at this time point, a temperature gradient was found between different vitreous compartments. There was a significant transvitreous temperature gradient (p < 0.001, rANOVA) from the lens toward the retina (with significant differences for all pairwise comparisons, p<0.05, Table 1). Posterior vitreous temperatures were higher than mid- and anterior vitreous temperatures. There was no association of baseline vitreous temperature with patient’s diagnosis, body temperature, blood pressure, heart rate or blood oxygen saturation (p > 0.05). Mean duration of vitrectomy was 6.4±0.75 minutes. Immediately after vitrectomy (time point 2), posterior, mid- and anterior vitreous temperatures were significantly lower compared to baseline (p < 0.001, Table 1). In addition, there was still a transvitreous temperature gradient (p < 0.001, Friedman test) from the lens toward the retina (with significant differences for all pairwise comparisons, p<0.05), and posterior vitreous temperatures were higher than mid- and anterior vitreous temperatures.
Table 1 presents mean temperature measurements for the three vitreous compartments at time points 1 and 2 for comparison of temperature changes. There were significant differences in post-vitrectomy temperature decreases between different compartments of vitreous (p < 0.001, Friedman test). The lowest temperature decrease (3.35±0.51 ˚С) was for the anterior vitreous, and the highest (3.8±0.59 ˚С), for the posterior vitreous (with significant differences for all pairwise comparisons, p<0.05). Next, we used regression analysis to identify the relationship of a change (Δ) in midvitreous temperature after vitrectomy with duration of additional surgical manipulations. There was a positive linear relationship between change in midvitreous temperature after vitrectomy and duration of additional surgical manipulations (r=0.64, p=0.002). Therefore, Δ index increased (at an average rate of 0.18 °С per minute) with an increase in duration of these manipulations. Compared to immediately after vitrectomy, the increase in midvitreous temperature after additional surgical manipulations was 2.21±1.11 °С, with a mean duration of these manipulations of 7.3±3.9 minutes. During and after vitreoretinal surgery, there were no complications that could be attributable to the introduction of additional probes into the vitreous. Discussion Monitoring of treatment parameters is of utmost importance in current medicine. Therapeutic controlled hypothermia has been successfully applied in various medical fields (like cardiac surgery, neurosurgery, resuscitation science, and neonatology) for improving brain cell resistance to ischemic conditions [11-15]. The ability to monitor temperature parameters under conditions of hypothermia in the human body or its parts plays a key role in achieving beneficial effects of hypothermia and reducing the risk of complications. The use of intraocular media and irrigation fluid temperature monitoring during vitreoretinal surgery is not common in current clinical practice. It is, however, well established that the temperature of the intraocular media approaches that of the body, and the temperature of irrigation solutions used during vitrectomy is commonly lower than that of the intraocular media, and conforms to the ambient temperature in the operating room [16]. Therefore, eye surgery is commonly performed under conditions of artificially induced local hypothermia, which was confirmed also by our findings. In the current study, we found statistically significant decreases in temperature in the each of the three compartments of the vitreous immediately after vitrectomy, with the lowest post-vitrectomy temperature of 30.1±0.45 °С recorded in the anterior vitreous. Previously, we have found that the use of a room-temperature infusion solution in vitreoretinal surgery in rabbits resulted in a drop of intraocular temperature to deep hypothermia levels [17]. It is noteworthy that, in the current study, the greatest temperature drop (3.8±0.59 °С) immediately after vitrectomy was observed in the posterior pretetinal vitreous, which is likely to be attributed to the direction of the fluid flow. In addition, an increase in the temperature in the vitreous was found after performance of additional surgical manipulations, which we believe to be due to a decrease in irrigation solution flow into the eye. Thus, with an increase in the duration of additional surgical manipulations, temperature in the vitreous increased at a rate of 0.18°C per minute. Moreover, in the current study, we demonstrated the presence of a transvitreous temperature gradient. Not surprisingly, at baseline, the highest temperature was recorded in the posterior vitreous (34.17±0.36 °С), evidencing the role of the choroid as a major source of heat in the human eye. These findings are in agreement with those of previous experimental in vivo studies [10]. In the current study, a transvitreal temperature gradient was found both before and after vitrectomy. Conclusion First, the presence of a transvitreous temperature gradient from the anterior toward the posterior vitreous of the human eye was confirmed. In a room with an ambient temperature of 24.4±0.51 °С, at baseline, the highest temperature (34.17±0.36 °С) was recorded in the posterior vitreous. Second, there were significant decreases in temperatures in vitreous compartments immediately after vitrectomy with irrigation solutions of 24.2±0.52 °С. In addition, at this time point, the lowest temperature of 30.1±0.45 °С was recorded in the anterior vitreous, whereas the greatest temperature decrease (3.8±0.59 ˚С) compared to baseline was for the preretinal posterior vitreous. Third, there were increases in temperatures in vitreous compartments after performance of additional surgical manipulations and, with an increase in the duration of these manipulations, temperature in the vitreous increased at a rate of 0.18°C per minute. Finally, eye surgery is commonly performed under conditions of artificially induced local hypothermia, warranting intraoperative monitoring of intraocular and irrigation fluid temperatures.
References 1.Postel EA, Pulido JS, Byrnes GA, et al. Long-term follow-up of iatrogenic phototoxicity. Arch Ophthalmol. 1998 Jun;116(6):753-7. 2.Farah M, Maia M, Rodrigues EB. Dyes in Ocular Surgery: Principles for Use in Chromovitrectomy. Am J Ophthalmol. 2009 Sep;148(3):332-40. 3.Hasumura T, Yonemura N, Hirata A, et al. Retinal Damage by Air Infusion during Vitrectomy in Rabbit Eyes. Invest Ophthalmol Vis Sci. 2000;41:4300–04. 4.Ocular perfusion pressure during pars plana vitrectomy: a pilot study. Rossi T, Querzoli G, Angelini G. Invest Ophthalmol Vis Sci. 2014 Dec 2;55(12):8497-505. doi: 10.1167/iovs.14-14493. 5.Zadorozhnyy OS, Nazaretian RE, Myrnenko VV, Naumenko VA, Maltsev EV, Pasyechnikova NV. Structure of the chorioretinal complex in the rabbit eye after vitrectomy. Report 1. Vitreous cavity irrigation with different temperature solutions for 30 minutes. J Ophthalmol (Ukraine). 2018;3:73-84. 6.Rinkoff J, Machemer R, Hida T, Chandler D. Temperature-dependent light damage to the retina. Am J Ophthalmol. 1986;102:452–62. 7.Tamai K, Toumoto E, Majima A. Local hypothermia protects the retina from ischaemic injury in vitrectomy. Br J Ophthalmol. 1997;81:789–94. 8.Romano MR, Romano V, Mauro A, et al. The effect of temperature changes in vitreoretinal surgery. Transl Vis Sci Technol. 2016 Feb 9;5(1):4. 9.Anatychuk LI, Pasyechnikova NV, Zadorozhnyy OS, et al. [A thermoelectric device for measuring intraocular temperature]. Thermoelektrika. 2015;3:31-40. Ukrainian. 10.Anatychuk LI, Pasyechnikova NV, Zadorozhnyy OS, et al. A thermoelectric device for and approaches to temperature measurement in various ocular compartments. J Ophthalmol (Ukraine). 2015;6:50-3. 11.Alzaga AG, Cerdan M, Varon J. Therapeutic hypothermia. Resuscitation. 2006 Sep;70(3):369-80. 12.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the ICU: Practical considerations, side effects, and cooling methods. Crit Care Med. 2009; 37:1101–20. 13.Saad H, Aladawy M. Temperature management in cardiac surgery. Glob Cardiol Sci Pract. 2013;2013:44–62. 14.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. 15.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012 Feb 22;13(4):267-78. 16.Iguchi Y, Asami T, Ueno S, et al. Changes in vitreous temperature during intravitreal surgery. Invest Ophthalmol Vis Sci. 2014 Apr 11;55(4):2344-9. 17.Zadorozhnyy OS, Nazaretian RE, Myrnenko VV, Naumenko VA, Pasyechnikova NV. [Experimental study of the epibulbar and intraocular temperature in the rabbit under hypothermic conditions]. Oftalmologiia. Vostochnaia Evropa. 2018;1:73-81. Russian.
|