J.ophthalmol.(Ukraine).2018;6:23-29.
|
http://doi.org/10.31288/oftalmolzh201862329 Received: 10 September 2018; Published on-line: 30 December 2018 Influence of local and systemic factors of type 2 diabetes mellitus on the functional status of platelets in patients with diabetic retinopathy and maculopathy S.Iu. Mogilevskyy1, Dr Sc (Med), Prof.; Yu.O. Panchenko1, Cand Sc (Med); S.V. Ziablitsev2, Dr Sc (Med), Prof.; D.S. Ziablitsev3, Cand Sc (Med) 1 Shupik National Medical Academy of Postgraduate Education; Kyiv (Ukraine) 2 Bogomolets National Medical University; Kyiv (Ukraine) 3 Kyiv Medical University; Kyiv (Ukraine) E-mail: sergey.mogilevskyy@gmail.com TO CITE THIS ARTICLE: Mogilevskyy SIu, Panchenko YuO, Ziablitsev SV, Ziablitsev DS. Influence of local and systemic factors of type 2 diabetes mellitus on the functional status of platelets in patients with diabetic retinopathy and maculopathy. J.ophthalmol.(Ukraine).2018;6:23-29. http://doi.org/10.31288/oftalmolzh201862329
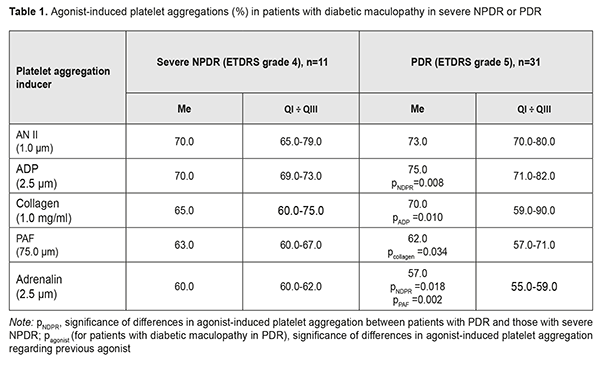
Background: To date, there is no information on relationships of systemic (activation of the sympathoadrenal and renin- angiotensin systems, SAS and RAS, respectively) and local (inflammation and remodeling of the retinal extracellular matrix and activation of the ocular purinergic system) mechanisms of the development of diabetic maculopathy (DMP) and diabetic macular edema (DME). Purpose: To identify the influence of systemic and local factors of type 2 diabetes mellitus (DM) on functional status of platelets in DMP and DME under conditions of severe nonproliferative (NPDR) or proliferative diabetic retinopathy (PDR). Materials and Methods: This study included 42 type 2 DM patients (42 eyes), of which 31 patients (31 eyes) and 11 patients (11 eyes) were found to have DMP in the presence of PDR and severe NPDR, respectively. Platelet aggregation in vitro to ADP, adrenaline, angiotensin 2 (Ang2), platelet activation factor (PAF) and collagen were assessed spectrophotometrically with a Chrono-Log aggregometer. Results: As platelet hyperreactivity to AN II, adrenalin, PAF and collagen were found in all study patients, activation of the RAS and SAS, and inflammation and remodeling of retinal extracellular matrix are non-specific pathogenetic mechanisms of DMP. Platelet reactivity to ADP was higher in PDR than in severe NPDR (р=0.008), which reflected the features of the pathogenesis of PDR. Development of DME in patients with DMP could be caused by a pronounced dysregulation of purinergic signaling in the eye, activation of the RAS and inflammation, which was reflected by platelet hyperreactivity to ADP, AN II and PAF, whereas a high platelet reactivity to collagen was characteristic of the absence of DME. Conclusion: The analysis of functional status of platelets allowed elucidating platelet activation mechanisms and identifying major platelet agonists that enabled platelet involvement in progression of DMP and development of DME in type 2 DM patients with proliferative diabetic retinopathy. Keywords: type 2 diabetes mellitus, diabetic maculopathy, diabetic macular edema, proliferative and non-proliferative diabetic retinopathy Introduction Under physiological conditions, the inner and outer blood-retinal barriers (BRB) protect the retina by regulating ion, protein, and water flux into and out of the retina, thus maintaining the homeostasis of ocular tissues [1]. In diabetic retinopathy, fluid accumulation takes place in the outer plexiform and inner nuclear layers, manifesting as swelling of Muller cells of the retina [2]. In addition, localized or diffused expansion of the retinal extracellular space can occur in the macular area. In this context, there is a need to elucidate the possible causes of increased BRB permeability in proliferative diabetic retinopathy (PDR) compared to nonproliferative diabetic retinopathy (NPDR), which results in swelling of, and damage to retinal nerve and glial cells, and may be the cause of a sudden or chronic loss of vision. Glycation end products [3], vascular endothelial growth factor (VEGF) [4], endothelin growth factor [5], activation of the sympathoadrenal system (SAS) [6] and renin-angiotensin system (RAS) [7] inflammation and retinal extracellular matrix remodeling [8] have been discussed as the factors causing this process. It should be noted that, in spite of active research in the field of pathogenesis of diabetic macular edema (DME), there are to date no effective treatment strategies for the pathology [9]. This is caused in part by the absence of rapid tests evaluating the influence of pathogenetic factors of diabetes mellitus (DM) on the BRB. Availability of such tests would allow identifying target alteration mechanisms and performing adequate medication correction of alterations. To date, there is no information on relationships of systemic (activation of the RAS and SAS) and local (inflammation and retinal extracellular matrix remodeling; activation of the ocular purinergic system) mechanisms of the development of diabetic maculopathy (DMP) and DME in PDR. Under these conditions, it is possible to establish neither the sequence of stages in the progression of ocular pathology nor major BRB alteration mechanisms. Systemic and local mechanisms of PDR pathogenesis are mediated by platelets and white cells which (1) have receptors to agonists present in circulating blood, and (2) can influence BRB permeability by secreting biologically active substances. Platelets play a major role here, they respond to adrenaline, angiotensin 2 (Ang2), platelet activation factor (PAF), collagen and cytokines [10] through increased functional activity [10]. As a result, platelets can influence BRB permeability directly (by secreting adenosine triphosphate (ATP), adenosine diphosphate (ADP), serotonin, and ions of Са2) and indirectly, through formation of platelet-and white cell aggregates under the influence of PAF that is secreted by neutrophils during the development of inflammation. These processes lead to activation of white cells, their adhesion to vessel endothelium and increase in ion and water flux [11]. Previously, we have reported on the functional status of platelets in patients with type 2 DM [12, 13] and in the presence of NPDR [14]. The purpose of the study was to identify the influence of systemic and local factors of type 2 DM on functional status of platelets in diabetic maculopathy and development of DME under conditions of severe nonproliferative or proliferative diabetic retinopathy. Materials and Methods This study included 42 type 2 DM patients (42 eyes), of which 31 patients (31 eyes) and 11 patients (11 eyes) were found to have DMP in the presence of PDR and severe NPDR, respectively, based on the combination of clinical and diagnostic examination findings and ETDRS classification in each case. DMP was diagnosed in the presence of specific diabetic retinal changes in the macular area, such as microaneurysms, hemorrhages, intraretinal microvascular abnormalities, and vitreoretinal vascular proliferation. Severity of DMP was graded as per the 2002 guidelines of the American Academy of Ophthalmology. DME was diagnosed in the presence of increase in retinal thickness in at least one of nine ETDRS subfields on Copernicus REVO SD OCT over reference database values (with yellow and red representing values increased with a significance of p <0.05, or p <0.01, respectively). Each patient underwent a routine eye examination including visual acuity, pneumotonometry, perimetry, gonioscopy, and keratometry and refractometry. In addition, ophthalmoscopy (Volk Super Field lens and Goldmann three-mirror contact lens), spectral domain OCT (Copernicus, Optopol Technologies, Zawierci, Poland; 3D and Raster scan types) and fundus photography (the ETDRS seven standard fields as per the modified ETDRS Airlie House classification) were performed. Platelets were isolated by centrifugation of plasma that had been isolated from patient’s citrated peripheral blood. Patients’ platelets were used to assess the functional activity of receptors. We used the following agonists involved in DM pathogenesis: (1) ADP which represents (a) the level of activation of purine receptors (P2Y12 and P2Y1 receptors) under the action of extracellulary purines, ATP and ADP, (b) secretory activity of platelets due to release of endogenous purines from dense granules, (c) potential for autocrine stimulation of platelet purine receptors; (2) adrenaline, a humoral factor whose level increases in stress response following activation of the SAS; (3) angiotensin II (AN-II), whose level increases following activation of the RAS; (4) platelet activation factor (PAF), a paracrine mediator that ensures both platelet stimulation and platelet-white blood cell interaction in inflammation; and (5) collagen, which reflects the results of extracellular matrix remodeling (increased blood collagen levels and/or expression of vascular basement membrane collagen). The agonists were obtained from Sigma (St. Louis, MO) and used in EC50 concentrations (adrenaline, 2.5±0.1 μm; collagen, 1.0±0.03 μg/ml; AN-II, 1.0±0.06 μm; PAT, 75.0±2.6 μm; and ADP, 2.5±0.05 μm) to produce 50% ± 5 % of the maximum rate of aggregation in healthy individuals. Platelet aggregation was assessed spectrophotometrically with a Chrono-Log aggregometer (Chrono-Log Corp, Havertown, PA). Informed consent was obtained in all cases. Statistical analysis was performed using Medcalc Software. The mean ( ) and standard deviation (±SD), or median (Me) and interquartile range (QI÷QIII) were calculated for the two groups. Statistical comparisons between the two groups were performed using a Student's t-test (for quantitative, normally distributed variables), a Wilcoxon rank-sum test (for quantitative, not normally distributed variables), and Fisher’s exact test. Differences were considered statistically significant at P < 0.05. Results and Discussion We found platelet hyperreactivity to all agonists under study (collagen, adrenaline, AN II, ADP and PAF) in patients with DMP in PDR versus controls (Table 1).
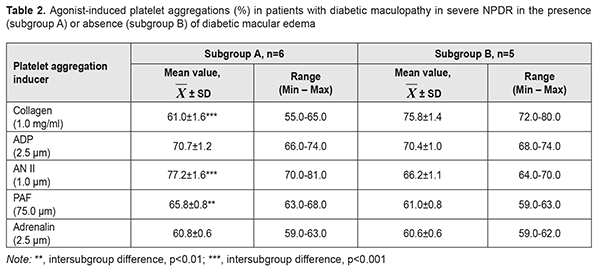
These patients demonstrated 7.1% increased platelet reactivity to ADP (p =0.008) and 5.0% decreased platelet reactivity to adrenalin (p ˂ 0.05) compared to those with DMP in severe NPDR. Platelet responses to AN II, collagen and PAF in the two groups were comparable and corresponded to hyperreactivity ranges (57-90%). Thus, as platelet hyperreactivity to AN II, adrenalin, PAF and collagen were found in DMP patients with severe NPDR and in those with PDR, activation of RAS and SAS, inflammation and remodeling of retinal extracellular matrix are non-specific pathogenetic mechanisms of DMP. An increased influence of ADP (р=0.008) on platelet aggregation was a feature of platelet reactivity in DMP patients with PDR compared to those with severe NPDR. This phenomenon reflected an increased stimulation of purine receptors, which may be considered as a factor of risk for DMP progression from severe NPDR to PDR. AN II and ADP were more potent inducers of platelet aggregation in DMP patients with PDR. Compared to ADP, other agonists, collagen, PAF and adrenalin, were 6.7%, 17.3% and 24.0%, respectively, less potent inducers of platelet aggregation (p ˂ 0.05 for all comparisons). Functional activities of АТ1 receptors and purine receptors were the highest, followed by GPVI receptor, PAF receptor, and α2-adrenoreceptor. In order to determine whether the functional activity of platelets in DMP in the presence of DME was different from that in the absence of DME, patients with NPDR were subdivided into two subgroups, the A subgroup comprising 6 patients (54.6%) with DME, and the B subgroup comprising 5 patients (45.4%) without any signs of DME (Table 2).
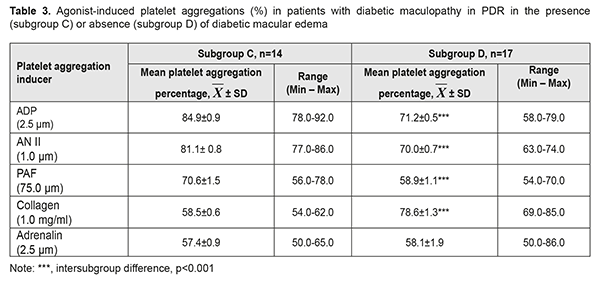
Patients of the A subgroup demonstrated platelet hyperreactivity to all agonists (had platelet aggregation >55%). Hyperreactivity of AT1 receptors for this subgroup ranged from 70% to 81%. Platelet response to AN II was 9.2% (р=0.009), 17.3% (р<0.001), 26.6% (р<0.001), and 27% (р<0.001) higher than those to ADP, PAF, collagen and adrenalin, respectively. Patents of the B subgroup demonstrated hyperreactivity of GPVI receptors to collagen, with collagen-induced platelet aggregation ranging from 72% to 80% (Table 2). Platelet response to collagen was 7.7% (р = 0.015), 14.5% (р < 0.001), 24.2% (р<0,001), and 25.1% (р<0.001) higher than those to ADP, AN II, PAF, and adrenalin, respectively. It was noteworthy that platelet responses to collagen, AN II and PAF in patients with DMP in severe NPDR varied depending on the presence or absence of DME. Thus, platelet responses to AN II and PAF collagen in the presence of DME were 16.6% higher (р<0.001) and 7.9% higher (р<0.01), respectively, than in the absence of DME, whereas platelet reactivity to collagen in the absence of DME was 24.3% higher (р<0.001), than in the presence of DME. The difference in platelet response to PAF between patients with DME and those without DME was statistically insignificant. Therefore, our comparison of functional activities of platelet receptors found that AN II and PAF reproduced platelet hyperreactivity and could be the cause of the development of DMN in patients with DMP in the presence of severe NPDR. Patients with PDR were subdivided into two subgroups, the C subgroup comprising 14 patients (45.2%) with DME, and the D subgroup comprising 17 patients (54.8%) without any signs of DME (Table 3).
Patents of the C subgroup demonstrated platelet hyperreactivity to all agonists, with ADP being the most potent collagen aggregation inducer, and with the activity of purine receptors to the agonist ranging from 78% to 92%. Platelet response to ADP was 4.7% (р = 0.011), 20.2% (р < 0.001), 45.1% (р<0.001), and 47.9% (р<0.001) higher than those to AN II, PAF, collagen, and adrenalin, respectively. Functional activity of purine receptors was the highest, followed by АТ1 receptor, PAF receptor, and GPVI receptor with α2-adrenoreceptor, respectively. As there was a high correlation between AN II-induced platelet aggregation and PAF-induced platelet aggregation, with Pearson r of.693 (р=0.006), the relationship between the RAS and inflammation was significant for the pathogenesis of DMN in patients with SMP in the presence of PDR. Patents of the D subgroup also demonstrated hyperreactivity of GPVI receptors to collagen, with collagen-induced platelet aggregation ranging from 69% to 85%. Platelet response to collagen was 10.4% (р < 0.001), 12.3% (р < 0.001), 33.4% (р<0.001), and 35.3% (р<0.001) higher than those to ADP, AN II, PAF, and adrenalin, respectively. Therefore, in patents of the D subgroup, functional activity of GPVI receptor was the highest, followed by purine receptors with АТ1 receptor, and PAF receptor with α2-adrenoreceptor, respectively. There were significant differences in platelet responses to collagen, ADP, AN II, and PAF between DMP patients in PDR in the presence and in the absence of DME. Thus, in the absence of DME, and irrespective of the stage of DR, there may could be extracellular matrix remodeling (overexpression of collagen and sulfated glycosaminoglycans [10]), which prevented accumulation of interstitial fluid and formation of DME. Platelet responses to ADP, AN II, PAF and collagen in the presence of DME were 19.2% higher (р<0.001), 15.9% higher (р<0.001), 19.9% higher (р<0.001) and 34.4% lower (р<0.001), respectively, than those in the absence of DME. Differences in platelet responses to adrenalin and ADP between patients with DME and those without DME were statistically insignificant. Therefore, the results of our comparison of the C and D subgroups with regard to functional activities of receptors suppose that ADP, AN II and PAF and could be indicators of the development of DME. At the same time, increased reactivity of platelet purine receptors to PAF reproduced the features of the pathogenesis of DMP in PDR. Therefore, our in vitro study of platelets has confirmed contributions of type 2 DM-associated systemic and local pathogenetic mechanisms to the development of SMP and DME. Increased platelet aggregation can lead to thrombus formation and retinal vessel hemorrhage. Consequently, functional activity of α2-adrenoreceptor and AT1, PAF and GPVI receptors of platelets might be an index for predicting the risk SMP in PDR. Less is known about a diagnostic value of the test of platelet stimulation with ADP. Our study found that agonist ADF-induced increased functional platelet activity is a universal mechanism involved in the pathogenesis of both DMP and DME. ATP and ADP secretion underpins ADP-induced platelet aggregation, and ATP and ADP not only stimulate purine receptors of the platelets proper (autocrine platelet stimulation through ADP) but also exert an effect on other cells (like endothelial cells, pericytes of blood-retinal barrier vessels, pigment epithelium cells, and nerve cells in the retina) that have appropriate receptors, P2X-, P2Y1- and P2Y12- [10]. In diabetic retinopathy, retinal levels of ATP, ADP and adenosine monophosphate are significantly increased [15]. Ecto-5'- nucleotidase (CF73), adenylate kinase 1 and nucleoside diphosphate kinase in the ocular structures contribute to maintaining the balance between pro-inflammatory ATP and anti-inflammatory adenosine. Immunohistochemical staining revealed selective expression of ecto-5'-nucleotidase (CF73) in retinal photoreceptor cells. Compared to patients with NPDR, those with PDR demonstrated higher adenylate kinase 1 activity and ATP levels [16]. Positive correlation between (1) intravitreal activity of adenylate kinase 1 and ATP and ADP levels, and (2) angiogenes (angiopoetin 1 and 2), profibrotic (TGFβ1) and proteolytic (matrix metalloproteinase 9) factors reflects a relationship of purinergic signaling in the eye with vasculogenesis and extracellular matrix remodeling. Retinal Muller cells release ATP that stimulates P2X7 receptors of endothelial cells, thus leading to their apoptosis [17]. Dysregulated calcium signaling triggered by overactivation of P2X7 receptors is a crucial step in the induction of neuronal and microvascular cell death in diabetes [18]. Hyperactivation of P2X7 receptors contributes to photoreceptor cell death, and age-related dysfunction and degeneration of retinal pigment epithelium. Purinergic signaling induces or modulates gliosis that is accompanied by deficient homeostatic support of neuronal networks. On the other hand, changes in glial metabolism lead to destruction of ATP and increased levels of adenosine that could provide neuroprotection of the retina. Modulation of purine receptors with medications, such as inhibition of P2X- and activation of adenosine receptors may has a clinical value in preventing apoptosis of photoreceptors, neuronal cells and pericytes in diabetic retinopathy. The adenosinergic system is widely regarded as a significant modulator of neurotransmission and the inflammatory response, through the action of the four types of adenosine receptors (A1R, A2AR, A2BR, and A3R) [19]. It has been experimentally demonstrated that the A3R selective agonist prevented apoptosis of retinal neural cells. [20]. The finding of hyperresponsiveness of purine P2Y1 and P2Y12 receptors to ADP in patients with PDR showed that autocrine platelet stimulation was pronounced, with effective functioning of extracellular signaling pathways. In patients with DMP in PDR in the presence of DME, platelet responsiveness to ADP was more pronounced than in patients without DME, which could evidence an increased dysregulation of purinergic signaling in ocular structures. This could lead to (a) impaired neurotransmission and the inflammatory response in the retina; gliosis; apoptosis of photoreceptors and microvascular cell death [21]; (b) increased extracellular levels of ATP and ADP, which cause increased BRB permeability (in the presence of activation of purine receptors on the surface of endothelial cells, of capillary wall pericytes and pigment epithelium cells) leading to further platelet stimulation; and (c) platelet preconditioning with thrombus formation and retinal vessel hemorrhage. Further research of purinergic signaling in ocular cells would open up opportunities for the development of novel therapeutic approaches to prevention and treatment of DMP and DME in proliferative diabetic retinopathy. Conclusion First, our in vitro study of platelets made it possible to analyze the functional activity of several receptors, and to identify the cluster of receptors reflecting the influence of pathogenetic factors of type 2 DM on target cells. This methodological approach allowed us to compare platelet activation mechanisms and identify major agonists providing for platelet involvement in (1) disease progression from severe non-proliferative diabetic retinopathy to proliferative diabetic retinopathy and (2) the development of diabetic macular edema. Second, the analysis of functional activity of platelet receptors in patients with diabetic macular edema in severe non-proliferative diabetic retinopathy versus proliferative diabetic retinopathy, and in proliferative diabetic retinopathy in the presence versus absence of diabetic macular edema demonstrated that activation of the renin-angiotensin and sympathoadrenal systems, development of inflammation and remodeling of retinal extracellular matrix were non-specific mechanisms of the pathogenesis of diabetic macular edema, whereas increased effect of ADP on platelets reflected a progression of edema. Platelet hyperreactivity to ADP, AN II and PAF may be markers of the development of diabetic macular edema, whereas platelet hyperreactivity to collagen was characteristic of the absence of diabetic macular edema. References 1.Willermain F, Scifp L, Weber C, Caspers L, Perret J, Delporte C. Potential interplay between hyperosmolarity and inflammation on retinal pigment epithelium in pathogenesis of diabetic retinopathy. Int J Mol Sci. 2018 Apr 2;19(4). pii: E1056. 2.Scholl S, Augustin A, Loewenstein A, Rizzo S, Kuppermann B. General pathophysiology of macular edema. Eur J Ophthalmol. 2011;21 Suppl 6:S10-9. 3.Xu J, Chen LJ, Yu J, Wang HJ, Zhang F, Liu Q, Wu J. Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell Physiol Biochem. 2018;48:705–17. 4.Urias EA, Urias GA, Monickaraj F, McGuire P, Das A. Novel therapeutic targets in diabetic macular edema: Beyond VEGF. Vision Res. 2017 Oct;139:221-7. 5.Sorrentino FS, Matteini S, Bonifazzi C, Sebastiani A, Parmeggiani F. Diabetic retinopathy and endothelin system: microangiopathy versus endothelial dysfunction. Eye (Lond). 2018;32(7):1157-1163. 2018 Jul;32(7):1157-1163. 6.Jiang Y, Zhang Q, Steinle JJ. Beta-adrenergic receptor agonist decreases VEGF levels through altered eNOS and PKC signaling in diabetic retina. Growth Factors. 2015;33(3):192-9. doi: 10.3109/08977194.2015.1054990. 7.Kim JH, Kim JH, Yu YS, Cho CS, Kim KW. Blockade of angiotensin II attenuates VEGF-mediated blood-retinal barrier breakdown in diabetic retinopathy. J Cereb Blood Flow Metab. 2009 Mar;29(3):621-8. doi: 10.1038/jcbfm.2008.154. 8.Dagher Z, Gerhardinger C, Vaz J, Goodbridge M, Tecilazich F, Lorenzi M. The increased transforming growth factor-β signaling induced by diabetes protects retinal vessels. Am J Pathol. 2017 Mar;187(3):627-638. doi: 10.1016/j.ajpath.2016.11.007. 9.Somilleda-Ventura SA, Garcia-Rubio YZ, Razo Blanco-Hernandez DM, Lima-Gomez V.[Association between visual improvement after photocoagulation and the use of angiotensin converting enzyme inhibitors in diabetic macular oedema]. Cir Cir. 2016 Jul-Aug;84(4):269-74. doi: 10.1016/j.circir.2015.09.004. Spanish 10.Barinov EF, Sulaieva ON, Gnilorybov AM. [Platelets]. Donetsk: Novyi Mir; 2012. Russian. 11.Ed Rainger G, Chimen M, Harrison MJ, Yates CM, Harrison P, Watson SP, et al. The role of platelets in the recruitment of leukocytes during vascular disease. Platelets. 2015 Aug 18; 26(6): 507–520. doi: [10.3109/09537104.2015.1064881] 12.Hudz AS, Mogilevskyy SIu, Maksymtsiv ML. Functional status of platelets in type 2 diabetes patients showing no diabetic fundus changes. J. Ophthalmol. (Ukraine). 2017;1:20-4. 13.Hudz AS, Maksymtsiv ML. [Platelets functional state and microcirculation disorders in patients with diabetes mellitus 2 type]. Arkhiv oftalmologii Ukrainy. 2017; 2(8):27-32. Ukrainian. 14.Hudz AS, Maksymtsiv ML, Ziablitsev SV, Mogilevskyy SIu. [Prothrombogenic phenotype of platelets in patients with nonproliferative diabetic retinopathy]. Mizhnarodnyi Endocrinologichnyi Zhurnal. 2018; 14 (2):doi: http://dx.doi.org/10.22141/2224-0721.14.2.2018.130558. Ukrainian. 15.Loukovaara S, Sahanne S, Jalkanen S, Yegutkin GG. Increased intravitreal adenosine 5'-triphosphate, adenosine 5'-diphosphate and adenosine 5'-monophosphate levels in patients with proliferative diabetic retinopathy. Acta Ophthalmol. 2015 Feb;93(1):67-73. doi: 10.1111/aos.12507. 16.Loukovaara S, Sandholm J, Aalto K, Liukkonen J, Jalkanen S, Yegutkin GG. Deregulation of ocular nucleotide homeostasis in patients with diabetic retinopathy. J Mol Med (Berl). 2017 Feb;95(2):193-204. doi: 10.1007/s00109-016-1472-6. 17.Portillo JC, Lopez Corcino Y, Dubyak GR, Kern TS, Matsuyama S, Subauste CS. Ligation of cd40 in human müller cells induces P2X7 receptor-dependent death of retinal endothelial cells. Invest Ophthalmol Vis Sci. 2016 Nov; 57(14): 6278–6286.doi: [10.1167/iovs.16-20301]. 18.Reichenbach A, Bringmann A. Purinergic signaling in retinal degeneration and regeneration. Neuropharmacology. 2016 May;104:194-211. doi: 10.1016/j.neuropharm.2015.05.005. 19.Vindeirinho J, Santiago AR, Cavadas C, Ambrósio AF, Santos PF. The adenosinergic system in diabetic retinopathy. J Diabetes Res. 2016;2016:4270301. doi: 10.1155/2016/4270301. 20.Galvao J, Elvas F, Martins T, Cordeiro MF, Ambrósio AF, Santiago AR. Adenosine A3 receptor activation is neuroprotective against retinal neurodegeneration. Exp Eye Res. 2015 Nov;140:65-74. doi: 10.1016/j.exer.2015.08.009. 21.Santilli F, Liani R, Di Fulvio P, Formoso G, Simeone P, Tripaldi R, et al. Increased circulating resistin is associated with insulin resistance, oxidative stress and platelet activation in type 2 diabetes mellitus. Thromb Haemost. 2016 Nov 30;116(6):1089-1099.
|