J.ophthalmol.(Ukraine).2018;6:71-75.
|
http://doi.org/10.31288/oftalmolzh201867175 Received: 13 September 2018; Published on-line: 31 December 2018 Genetic susceptibility to the development of primary open angle glaucoma A.M. Sergienko1,2, Dr Sc (Med), Prof.; V.O. Melnik3, Chief Physician, Cand Sc (Med); M.V. Khoroshkova1, Ophthalmologist Received: 13 September 2018 Published: 31 December 2018 1 Professor Sergienko Eye Clinic; Vinnytsia (Ukraine) 2 Vinnytsia National Pirogov Memorial Medical University; Vinnytsia (Ukraine) 3 Visiobud Plus Clinic; Vinnytsia (Ukraine ) E-mail: margulichka85@gmail.com TO CITE THIS ARTICLE: Sergienko AM, Melnik VO, Khoroshkova MV. Human body donation in foreign legislations. J.ophthalmol.(Ukraine).2018;6:71-75. http://doi.org/10.31288/oftalmolzh201867175
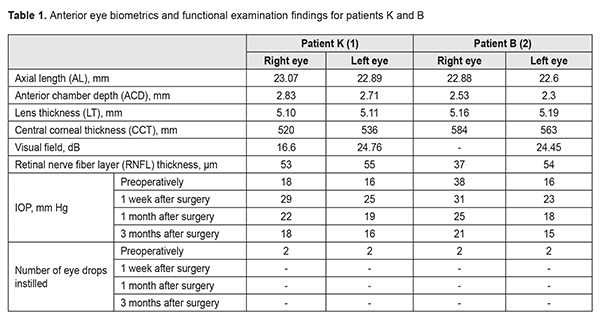
Background: Primary open angle glaucoma is a multifactorial optic neuropathy and associated with a number of factors such as age, ethnicity, family history, central corneal thickness, and intraocular pressure (IOP). In addition, it might be associated with myopia and certain systemic disorders. Family history is the highest risk factor as shown by the presence of glaucoma in first-degree relatives. Purpose: To assess the potential for the development of glaucoma due to genetically predisposed features of ocular anatomy and age-related changes in these features based on a case glaucoma in monozygotic siblings. Materials and Methods: We analyzed the clinical case of two monozygotic sisters with the disease based on complaints, history, physical and instrumental examination, treatment and outcomes. Results: The two monozygotic sisters (1) were diagnosed with the same disorder approximately at the same age, (2) presented with similar ocular biometric characteristics and age-related changes of the anterior eye, and (3) underwent the same surgery on both eyes, which resulted in improved visual functions, stable IOP reduction, stable perimetric visual fields, and stable OCT derived RNFL thickness. Conclusion: The results obtained in monozygotic siblings confirm that genetic susceptibility to changes in anterior eye parameters and age-related changes in anterior ocular structures along with environmental factors constitute the main cause of hydrodynamic impairment resulting in the development of glaucoma. Keywords: open angle glaucoma, anterior chamber depth, lens thickness, corneal thickness, pseudoexfoliation syndrome Introduction Glaucoma is the second most common global cause of blindness after cataract. It is projected that the number of people with glaucoma will increase to 79.7 million by 2020, and of these, 74% will have open angle glaucoma (OAG) [1]. In addition, bilateral blindness will be present in 5.9 million people with OAG and 5.3 million people with angle closure glaucoma (ACG). Epidemiological studies suggest that by 2040 the prevalence of primary open angle glaucoma (POAG) is estimated to increase to 111.8 million due to population aging [1-3]. Glaucoma comprises a group of heterogeneous progressive optic neuropathies characterized by morphological changes in the optic nerve and retinal nerve fiber layer (RNFL). These changes are associated with progressive damage of retinal ganglion cells and visual field loss. OAG comprises the majority of cases in the United States and Western Europe, of which, POAG is the most common type. The disease is multifactorial and associated with a number of factors such as age, ethnicity, gender, central corneal thickness, intraocular pressure (IOP), myopia, and genetic factors. In addition, it might be associated with systemic disorders [2, 4]. Genetic population studies have confirmed that family history is a major risk factor for glaucoma [5, 6]. The action of a large number of grouped or independently inherited genes along with environmental factors results in anatomical abnormalities of the anterior eye and development of glaucoma. Such abnormalities include adhesion of the iris to the anterior lens capsule due to lens thickening with advancing age. This leads to abnormal aqueous outflow between these structures, and increased pressure in the posterior chamber, resulting in elevated IOP and glaucoma. Assessment of anterior eye structures is an essential part of the examination of the eye. This assessment can be performed with a number of modalities such as A-scan ultrasound biometry, ultrasound biomicroscopy (UBM) and gonioscopy. In addition, novel anterior eye imaging techniques like anterior segment optical coherence tomography (ASOCT) provide both for quantitative assessment and good visualization. It has been demonstrated that the higher preoperative IOP and the more marked the changes in the anterior chamber of the eye (the shallower the anterior chamber), the more marked the postoperative IOP changes [7-10]. Ocular biometric characteristics are known to vary with ethnicity [11]. Knowledge of normal changes in ocular anatomy can aid in our understanding of the pathogenesis, diagnosis and management of glaucoma. Previous epidemiological studies have demonstrated important relationships between ocular biometric characteristics and the ocular disorder. Alaskan Eskimos, who were shown to have a markedly shallower anterior chamber than Chinese, Blacks and Whites, are particularly prone to glaucoma. Long axial length in Asian peoples has been consistently associated with a high prevalence of myopia in Asian populations [12]. Findings of these and numerous other studies might implicate that there is a relationship between the prevalence of glaucoma and ocular biometric characteristics. Glaucoma is thought to be very rare in Māori and Pacific people of New Zealand, and the deeper anterior chamber identified in the study by Yoon et al [12] may have a protective role against the development of glaucoma. In a recent epidemiological study carried out in Norfolk Island, a small Pacific island situated between New Zealand and Australia, ocular biometric parameters were compared between Norfolk Island Pitcairn Pedigree (those who could trace their family history to the indigenous Polynesian island inhabitants) and non‐Pitcairn Pedigree who were mostly composed of Caucasians. This study did not find any significant difference in biometric parameters between the two populations, except for intraocular pressure and mean keratometry value. Genetic analysis has shown that people belonging to the Norfolk Island Pitcairn Pedigree were genetically 88% European and 12% Polynesian [12]. Numerous studies have confirmed relationships between preoperative characteristics (anterior chamber depth and IOP) and changes in IOP after cataract-only surgery or combination glaucoma-plus-cataract surgery [7-10]. Genetic susceptibility is due to multiple genes, and less than 10% of cases of glaucoma are caused by specific gene mutations. Among these genes are TIGR/myocilin, CYP1B1 and Optineurin or OPTN. POAG patients with myocilin mutations generally have juvenile or early-adult-onset form of glaucoma. Mutations in CYP1B1 have been mainly reported in congenital glaucoma. Optineurin is primarily responsible for cases of familial normal tension glaucoma [2, 4, 6]. Data from the Baltimore Eye Survey and Barbados Eye Study have been used to examine the association of POAG with a positive family history of glaucoma, and it was found that age-adjusted associations of POAG with a history of glaucoma were higher in siblings than in parents or children [3, 15, 17]. Another population-based study found that glaucoma occurred in 10.4% of glaucoma patients’ siblings, and the disease tended to occur more frequently in monozygotic siblings [2]. Screening targeted at individuals with a family history of glaucoma will contribute to early diagnosis of the disease in high risk groups. Adequate management and follow-up of such patients will prevent blindness in them The purpose of the study was to assess the potential for the development of glaucoma due to genetically predisposed features of ocular anatomy and age-related changes in these features based on a case glaucoma in monozygotic siblings. Materials and Methods Two twin sisters (K and B, born in 1948) presented to the private eye clinic with complaints of gradually decreasing vision in both eyes in January 2018. They had a medical history significant for glaucoma (diagnosed 5 years earlier), for which they had been receiving eye drops. Since 2013 to 2018, they visited the eye clinic several times, and their vision had been gradually decreasing in both eyes, with a gradual visual field loss detected by perimetry. They had positive family history of glaucoma (their brother, born in 1954, was affected) and glaucoma blindness (their father went blind with glaucoma combined with cataract). Patient K (born in 1948): On examination, uncorrected visual acuity (UCVA) was 0.3 OD and 0.2 OS, best-corrected visual acuity (BCVA) was 1.0 with -3.75D sph OD and 0.8 with -4.0D sph OS, and IOP was 18 mmHg OD and 16 mmHg OS. Gonioscopy of both eyes revealed narrow anterior chamber angles with mixed pigmentation. Anterior eye biometrics and functional examination findings are presented in the Results section. The following diagnosis was formulated based on patient’s complaints, history, physical and instrumental examination: complicated cataract and severe open-angle glaucoma (with moderately increased IOP managed with eye drops) associated with pseudoexfoliation syndrome OD; complicated cataract and moderate open-angle glaucoma (with moderately increased IOP managed with eye drops) associated with pseudoexfoliation syndrome OS. The patient was recommended to have combined surgery for cataract and glaucoma in the right eye. She underwent phacoemulsification with implantation of IOL in combination with glaucoma surgery (modified tunnel trabeculopuncture) in the right eye on January 31, 2018. Patient B (born in 1948): On examination, UCVA was 0.01 OD and 1.0 OS, and IOP was 28 mmHg OD and 16 mmHg OS. Gonioscopy of both eyes revealed narrow anterior chamber angles with mixed pigmentation. Characteristics of the anterior segment and functional examination findings are presented in the Results section. The following diagnosis was formulated based on patient’s complaints, history, physical and instrumental examination: complicated cataract and terminal open-angle glaucoma (with elevated IOP managed with eye drops) associated with pseudoexfoliation syndrome OD; complicated cataract, pseudoexfoliation syndrome, and moderate open-angle glaucoma (with moderately increased IOP managed with eye drops) associated with pseudoexfoliation syndrome OS. The patient was recommended to have combined surgery for cataract and glaucoma in the right eye. She underwent phacoemulsification with implantation of IOL in combination with glaucoma surgery (modified tunnel trabeculopuncture) on the right eye on January 31, 2018. All surgery was conducted by one surgeon (VOM). Surgical technique The dissection of the conjunctiva was performed with a limbus-based approach, and the conjunctiva and Tenon capsule were separated from the sclera. The Tenon capsule was removed. A one-half thickness trapezoidal limbus-based scleral flap (approximately 4 x 5-mm long) was outlined. A one-third thickness triangular limbus-based scleral flap was then outlined and dissected beneath the trapezoidal flap. The outer wall of Schlemm’s canal was removed. A routine phacoemulsification with IOL implantation was performed, which featured methyl blue staining and maintenance of remnants of the anterior wall of the lens vesicle. A microspatula was used to puncture the inner Schlemm’s canal wall (tunnel trabeculopuncture) in the area of the shaped scleral bed sideways from the main filtration site. Lens vesicle remnants were introduced into Schlemm’s canal lumen. A suture was placed through the superficial sclera flap. During the early follow-up, glaucoma eye drops were withdrawn with regard to the operated eye. Patients were recommended (a) to have follow-up visits at 1 week, 1 month and 3 months, and (b) to undergo phacoemulsification with implantation of IOL in combination with glaucoma surgery (modified tunnel trabeculopuncture) on the fellow eye. Both patients had their fellow eyes operated on in a month. Results The case is interesting in that twin sisters were diagnosed with the same disorder approximately at the same age, and presented with similar ocular biometric characteristics and age-related changes of the anterior eye (axial length and corneal thickness measurements, shallow anterior chamber, thickened lens, pseudoexfoliation, and similar gonioscopy measurements and OCT derived RNFL thickness). In addition, both eyes of both patients underwent combination surgery that resulted in improved visual acuity, stable IOP reduction, stable perimetric visual fields, and stable OCT derived RNFL thickness. During the early follow-up, glaucoma eye drops were withdrawn with regard to the operated eye. OCT and automated perimetry revealed that glaucoma remained stable during the late follow-up period. Genetic susceptibility to short axial length, thin central corneal thickness, shallow anterior chamber depth, and thick crystalline lens, along with age-related changes in the eye and environmental factors constitute the main cause of raised IOP, which results in glaucoma. Table 1 presents characteristics of the anterior segment and functional examination findings.
The anterior segment data obtained demonstrate that age-related changes bear the genetic basis often manifesting as low aqueous outflow facility, elevated IOP, and, as a result, the development of glaucoma. Therefore, early cataract surgery (in increased lens size, decreased anterior chamber volume, and pseudoexfoliation syndrome) is a pathogenetically treatment for the disease. Conclusions Age-related ocular changes in the presence of genetic factors in the twin sisters resulted in the identical type of glaucoma, which was treated by the identical type of surgery with an identical postoperative restoration process. First, this case exemplifies that genetic-related ocular anatomic features and age-related changes in anterior ocular structures (decreased anterior chamber volume, increased lens size, and changes in corneal thickness) constitute the main cause of hydrodynamic impairment and elevated IOP resulting in the development of glaucoma. Second, detection of changes in anterior ocular structures (decreased anterior chamber volume, increased lens size, and changes in corneal thickness) in the presence of pseudoexfoliation syndrome is a rationale for surgical treatment (phacoemulsification with implantation of IOL). Finally, detection of changes in anterior ocular structures (decreased anterior chamber volume, increased lens size, and changes in corneal thickness) in the presence of pseudoexfoliation syndrome and clinically detected glaucoma is a rationale for combination glaucoma-plus-cataract surgical treatment (phacoemulsification with implantation of IOL plus glaucoma surgery).
References 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006 Mar;90(3):262-7. 2.Amero K, Kondkar AA, Chalam KV. An Updated Review on the Genetics of Primary Open Angle Glaucoma. Int J Mol Sci. 2015 Dec 4;16(12):28886-911. 3.Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014 Nov;121(11):2081-90. 4.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–24. 5.Tomlinson A, Leighton DA. Ocular dimensions in the heredity of angle-closure glaucoma. Br J Ophthalmol. 1973 Jul;57(7):475-86. 6.Gramer G, Weber BH, Gramer E. Results of a Patient-Directed Survey on Frequency of Family History of Glaucoma in 2170 Patients. Invest Ophthalmol Vis Sci. 2014 Jan 13;55(1):259-64. 7.Siak J, Quek D, Nongpiur M, et al. Anterior Chamber Angle and Intraocular Pressure Changes After Phacoemulsification: A Comparison Between Eyes With Closed-angle and Open-angle Glaucoma. J Glaucoma. 2016 Mar;25(3):e259-64. 8.Masis Solano M, Lin SC. Cataract, phacoemulsification and intraocular pressure: Is the anterior segment anatomy the missing piece of the puzzle? Proq Retin Eye Res. 2018 May;64:77-83. 9.Moghimi S, Abdi F, Latifi G, et al. Lens parameters as predictors of intraocular pressure changes after phacoemulsification. Eye (Lond). 2015 Nov;29(11):1469-76. 10.Yang HS, Lee J, Choi S. Ocular biometric parameters associated with intraocular pressure reduction after cataract surgery in normal eyes. Am J Ophthalmol. 2013 Jul;156(1):89-94.e1. 11.Salmon JF, Swanevelder SA, Donald MA. The Dimensions of Eyes with Chronic Angle-closure Glaucoma. J Glaucoma. 1994 Fall;3(3):237-43. 12.Yoon JJ, Misra SL, McGhee CNj, Patel DV. Demographics and ocular biometric characteristics of patients undergoing cataract surgery in Auckland, New Zealand. Clin Exp Ophthalmol. 2016 Mar;44(2):106-13. 13.Hyman L, Wu SY, Connell AM, et al. Prevalence and causes of visual impairment in the Barbados Eye Study. Ophthalmology. 2001 Oct;108(10):1751-6. 14.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991 Nov 14;325(20):1412-7. 15.Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991 Jul 17;266(3):369-74. 16.Kim M, Park KH, Kim TW, et al. Anterior chamber configuration changes after cataract surgery in eyes with glaucoma. Korean J Ophthalmol. 2012;26:97–103. 17.Leske MC, Wu SY, Honkanen R, et al. Nine-year incidence of open-angle glaucoma in the Barbados Eye Studies. Ophthalmology. 2007 Jun;114(6):1058-64. 18.Sihota R, Ghate D, Mohan S, et al. Study of biometric parameters in family members of primary angle closure glaucoma patients. Eye (Lond). 2008;22:521–527. 19.Sun JH, Sung KR, Yun SC, et al. Factors associated with anterior chamber narrowing with age: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2012 May 9;53(6):2607-10.
|