J.ophthalmol.(Ukraine).2018;5:45-48.
|
https://doi.org/10.31288/oftalmolzh201854548 Received: 03 August 2018; Published on-line: 26 October 2018 Polymorphism of TGF-β1 (rs1800469) in children with different degrees of myopia N.V. Malachkova, Cand Sc (Med); D.A. Yatsenko, Postgraduate Student; G.P. Ljudkevich; V.M. Shkarupa, Cand Sc (Biol) Vinnytsia National Pirogov Memorial Medical University; Vinnytsia (Ukraine) E-mail: malachkovanataliia@gmail.com, dr.yatsengo@gmail.com TO CITE THIS ARTICLE: Malachkova NV, Yatsenko DA, Ljudkevich GP, Shkarupa VM. Polymorphism of TGF-β1 (rs1800469) in children with different degrees of myopia. J.ophthalmol.(Ukraine).2018;5:45-48. https://doi.org/10.31288/oftalmolzh201854548
Background: TGF-β is a key intrascleral mediator of extracellular matrix remodeling. Purpose: To investigate the pattern of allele frequency and genotype distribution for TGF-β1 gene rs1800469 among the Ukrainian Podillia region’s pediatric population with different degrees of myopia. Materials and Methods: Real-time polymerase chain reaction was used for genotyping for TGF-β1 gene rs1800469 in 105 children (210 eyes) with different degrees of myopia and 107 emmetropic children. Results: Compared to controls, significant differences in allele and genotype frequencies for the SNP under investigation were found only in the high myopia group. The presence of the C allele of TGF-β1 -509 C>T (rs1800469) was found to increase the risk for developing high myopia (OR = 2.44, 95% CI, 1.17–5.08; p = 0.02). The presence of a variant T allele was found to have an additive protective effect against developing high myopia in the CT genotype carriers (OR = 0.85, 95% CI, 0.34–2.16; p = 0.02) and TT genotype carriers (OR = 0.16; 95% CI, 0.02–1.29; p = 0.02). Conclusion: To our best knowledge, this study is the first to demonstrate that the CC genotype of rs1800469 is associated with the risk of the development of high myopia in a European population. Keywords: myopia, sclera, TGF-β1, polymorphism
Introduction Myopia is a common multifactorial condition that has a strong inherited component. It is estimated that by 2050, 49.8% of the world population will have myopia, and the percentage of individuals with high myopia will increase from 2.7% to 9.8% of the world population [1]. Progressive disease results in severe complications like glaucoma, degenerative changes and detachment of the vitreous and retina, and is characterized by an alteration in ocular function which substantially affects professional and social adaptation of myopic adolescents and quality of life of myopes [2]. Myopia is commonly the result of abnormal elongation of the eye, which may be genetically mediated and progress in adolescence [3]. Studies in animal myopia models have demonstrated that increased scleral matrix remodeling can lead to exaggerated eye growth causing myopia [4-5]. Changes in the transforming growth factor (TGF)-β isoform profile result in an altered scleral extracellular matrix (ECM) composition. TGF-β1, one of the three isoforms of TGF-β, stimulates the production of ECM proteins, collagen and fibronectin, and reduces the secretion of ECM degradation enzymes, collagenase, heparinase and stromelysin, or stimulates the production of proteins that inhibit their activity, tissue plasminogen activator-1 and tissue inhibitor of metalloproteinases [4-8]. Although some studies [9-12] have reported on the association of TGF-β1 single nucleotide polymorphisms (SNPs) with high myopia, others [11-14] have either failed to reveal the association or reported inconsistent results on the subject. It should be, however, noted that studies on the potential value of TGF-β1 SNP in the pathogenesis of myopia have been conducted only among Asian populations, and, of these studies, all except one [14] were conducted exclusively among high myopes. It is not established whether there is an association between TGF-β1 SNP and myopia of different degrees among European populations. Understanding the scleral molecular mechanisms underlying ocular growth and resulting in the development of refractive errors, particularly axial myopia is essential to identifying plausible therapeutic targets in the corneoscleral tunic [4]. The purpose of the study was to investigate the pattern of allele frequency and genotype distribution for TGF-β1 gene rs1800469 among the Ukrainian Podillia region’s pediatric population with different degrees of myopia. Materials and Methods One hundred and five myopic children (210 eyes; mean age, 11.3±3.4 years) from various oblasts (like Vinnytsia, Khmelnytskyi, Cherkasy, Odesa, Kirovohrad, and Kyiv oblasts) of the Ukrainian Podillia region who were under our observation during 2016 to 2017 underwent genotyping for TGF-β1 gene rs1800469. Patients were divided into the low, moderate and high myopia groups with 49, 34, and 22 individuals, respectively. The study was conducted at the Regional Clinical Pediatric Hospital and OPTIMAL eye care center, Vinnytsia. The control group included 107 emmetropic children (214 eyes; age, 4 to 13 years; mean age, 7.7±1.3 years). This study followed the ethical standards stated in the Declaration of Helsinki and was approved by the Local Ethics Committee of the Vinnytsia National Pirogov Memorial Medical University. Written informed consent was obtained from all individual participants included in the study. Human genomic DNA was extracted from buccal epithelial cells and purified using the NeoPrep DNA Magnet Extraction Kit (Neogen, Ukraine) according to the manufacturer’s instructions. Real time polymerase chain reaction (PCR) was performed by using a TGF-β1 RT-PCR Assay (GenoTekhnologiya, Russia) on an iCycler iQ5™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. The chi-square test was used to test genotype distributions for deviation from Hardy-Weinberg equilibrium. The odds ratios (OR) with 95% confidence intervals (CI) were calculated to estimate the degree of association between the TGF-β1 -509 C>T variant and the risk of myopia. Potential associations of the SNP with the risk of myopia were assessed using logistic regression under dominant, recessive and additive models of inheritance. Genotype frequencies were compared between groups using a chi‐square test, or, when at least one cell had an expected frequency of ≤5, Fisher's exact test. Results and Discussion The genotyping results are presented in Table 1.
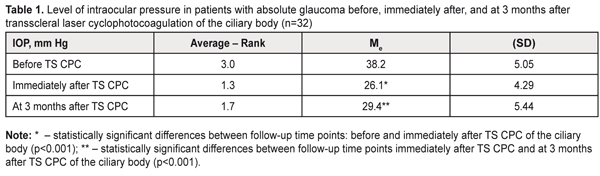
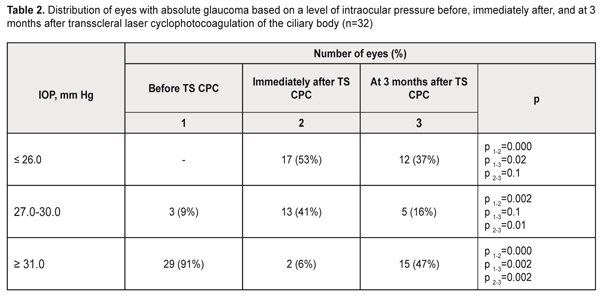
Genotype distribution followed the Hardy–Weinberg equilibrium in all groups except the moderate myopia group (χ2 = 6.8; р = 0.01). In the low myopia group, the allele frequency and genotype distribution for TGF-β1 gene rs1800469 were identical to those seen in the control group. The frequency of the variant T allele tended to be higher, and that of homozygous C allele carriers tended to be lower in moderate and high myopes, compared to emmetropes and low myopes (Table 1). However, compared to controls, significant differences in allele and genotype frequencies for the SNP under investigation were found only in the high myopia group (Table 2).
In this group, the frequency of the variant T allele was 1.8-fold higher than in the low myopia group (p = 0.02) and the control group (p = 0.01). The frequency of the T allele in the high myopia group (0.25) was 1.6-fold lower than in the moderate myopia group (0.40), although the difference was not statistically significant (p = 0.11). The presence of the C allele of TGF-β1 -509 C>T SNP (rs1800469) was found to increase the risk of high myopia (OR = 2.44, 95% CI, 1.17–5.08; p = 0.02; Table 2). Analysis of the additive model of inheritance demonstrated the additive nature of the effect of genotypes and alleles of TGF-β1 rs1800469 on the risk of high myopia (Table 2). Thus, homozygous CС carriers had an increased risk of high myopia (OR = 2.47; 95% CI, 0.97–6.27; p = 0.02). The presence of one variant T allele in heterozygous carriers was found to decrease the risk of high myopia (OR = 0.85, 95% CI, 0.34–2.16; p = 0.02), whereas the presence of one variant T allele in homozygous carriers was found to have a clear protective effect against this pathology (OR = 0.16; 95% CI, 0.02–1.29; p = 0.02). The increased risk of high myopia in allele C carriers and CC genotype carriers (р = 0.05) was present in both dominant and recessive models due to the additive effect of alleles. No association of TGF-β1 -509 C>T SNP (rs1800469) with the risk of moderate or low myopia was present in any of the models under investigation. Analysis of the study findings requires considering the functional significance of the SNP under investigation. The common TGF-β1 promoter SNP c.-1347C > T (-509C-T, rs1800469) has been linked to a nearly twofold difference in plasma levels among individuals and with risk, progression, and outcome of numerous diseases. The difference in TGF-β1 levels is due to transcriptional suppression by activator protein 1 (AP1) binding to the TGFB1 promoter. An AP1 complex containing JunD and c-Fos binds to the promoter region of TGFB1 only in the presence of the C allele. Increased TGF-β1 levels are often associated with the -509T allele because of the loss of negative regulation by AP1 [15-17]. TGF-β1 levels have been found to be lowest in the CC genotype carriers, followed by the heterozygote carriers, and highest in the TT genotype carriers [17]. Axial myopia results from increase in anteroposterior length of the eye, which is accompanied by elongation and thinning of the sclera. Scleral thickness in eyes with low myopia is close to that in emmetropic eyes, but is markedly decreased in eyes with high myopia [18]. The above processes result from biochemical changes that are controlled by the TGF-β1 signaling pathways. TGF-β1 has been shown to be expressed in the sclera, and to be capable of up-regulating collagen synthesis in scleral fibroblasts in a dose-dependent manner [8]. Decreased TGF-β1 expression levels have been observed in experimental myopia in animals, with 30% changes in TGF-β1 levels causing an approximately 15% reduction in collagen synthesis [7]. In addition, scleral expression levels of the TGF-β isoforms were differentially reduced in a time-dependent manner, possibly reflecting TGF-β-specific roles in the remodeling of the scleral ECM at different stages of myopia development [7]. Furthermore, as mentioned above, TGF-β1 decreases the secretion of enzymes that inhibit ECM degradation, and stimulates the production of proteins that inhibit their activity [4-8]. This suggests that individuals with genetically determined low TGF-β1 levels are prone to the development of high myopia. In low myopia, with subtle scleral thickness changes (due to reduced collagen synthesis and activities of ECM-destructive enzymes that are controlled by the TGF-β1 signaling pathways), other etiologic factors than TGF-β1 expression may play a key role in the pathogenesis. Regeneration of scleral tissue is expected as promising future therapy; however, the number of studies conducted in this area is limited [7-9]. Nevertheless, targeted delivery of TGF-β1 to the sclera could be considered as an option for the prevention of progressive myopia. A number of areas of further research have been identified that would develop the mechanisms for the delivery of such therapy and elucidate possible side effects [19]. The findings of the current study (a) demonstrate that it is reasonable to use genotyping of TGF-β1 rs1800469 for early diagnosis of the risk of high myopia, and (b) warrant further research on the potential for application of the TGF-β1 signaling pathways for the development of prospective methods for prevention and treatment of complications of myopia. Conclusion To our best knowledge, the current study is the first to demonstrate that the CC genotype of rs1800469 is associated with the risk of the development of high myopia among a European population (OR = 2.47, 95% CI, 0.97–6.27, р = 0.02). There were no statistically significant differences in allele frequencies or genotype distribution for the SNP between emmetropic children and children with low or moderate myopia.
References
|