J.ophthalmol.(Ukraine).2018;5:39-44.
|
https://doi.org/10.31288/oftalmolzh201853944 Received: 16 July 2018; Published on-line: 26 October 2018 Clinical efficacy of combination treatment for advanced-stage axial diabetic optic neuropathy M.A. Karliychuk, Cand Sc (Med) Higher State Educational Establishment of Ukraine «Bukovinian State Medical University»; Chernivtsi (Ukraine) E-mail: mari13karli@gmail.com TO CITE THIS ARTICLE: Karliychuk MA. Clinical efficacy of combination treatment for advanced-stage axial diabetic optic neuropathy. J.ophthalmol.(Ukraine).2018;5:39-44. https://doi.org/10.31288/oftalmolzh201853944 Background: There is no standard of therapy for diabetic optic neuropathy (DON) which takes into consideration the type and stage of the disease. Purpose: To assess the clinical efficacy of combined adjunct treatment with thioctic acid, a combination of vitamins В1, В6, and В12, ethyl methyl hydroxypyridine succinate, citicoline and brimonidine tartrate in the management of advanced-stage axial DON. Materials and Methods: Forty patients (63 eyes) were followed up after being diagnosed with advanced-stage axial DON. The adjunct group was composed of 20 patients (31 eyes) who were administered two courses a year of the following treatment as a combined adjunct to hypoglycemic therapy: 1) a 600 mg intramuscular injection of thioctic acid (Berlithion), daily for 21 days, followed by switching to oral regimen, at a dose of one 300 mg tablet twice a day for 21 days, (2) a 2 ml intramuscular injection of Milgamma, once per 3 days for 21 days, followed by switching to oral regimen, one tablet thrice a day for 21 days, (3) a 100 mg intramuscular injection of ethyl methyl hydroxypyridine succinate (Armadine), twice a day for 14 days, and (4) a 500-mg intravenous bolus of citicoline (Ceraxon), twice a day for 14 days, followed by switching to oral regimen, 500 mg daily for a month. In addition, they were administered topical brimonidine tartrate 0.2%, 1-2 drops twice a day on a constant basis. The control group (20 patients; 32 eyes) received hypoglycemic therapy only. In addition to routine eye examination, retinal and optic nerve optical coherence tomography and electrophysiology were performed. Patients underwent an examination at baseline and 1.5, 6, 7.5, 12, 13.5, 24 and 25.5 months after treatment. Results: The combined adjunct treatment was found to result in improvements in study indices in 83.9% of patients of the adjunct group, with 191% better visual acuity (p < 0.05), 235.0% lower electrically evoked phosphene thresholds (p < 0.05), 38.5% less ganglion cell complex (GCC) focal loss volume (FLV), and 36.5% less thickness of the lamina cribrosa compared to controls at 25.5 months. Conclusion: Our combination treatment was found to be clinically efficacious in improving structural and functional characteristics of the optic nerve in patients with advanced-stage axial DON. Keywords: axial diabetic optic neuropathy, advanced stage, comprehensive treatment, efficacy
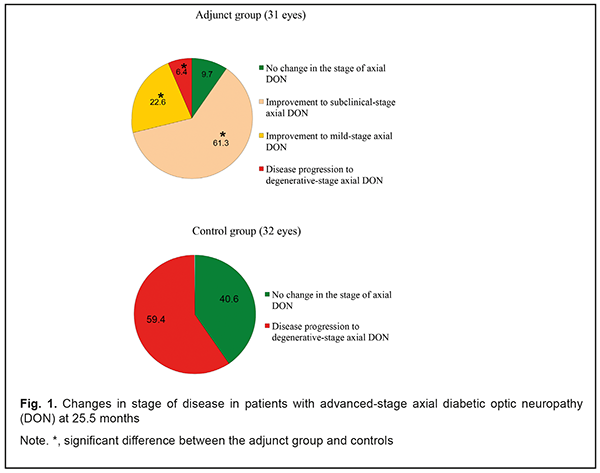
Introduction Thirty to 50 percent of patients with diabetes mellitus (DM) will eventually develop damage to the nerves of the peripheral nervous system during the course of their disease [1]. Metabolic and vascular factors are not the only factors that have been implicated in the pathogenesis of diabetic polyneuropathy (DPN) [2]. Despite intense research on the consequences of hyperglycemia on nerve functions, the biological mechanisms underlying DPN are still largely unknown, and treatment options lacking [3-4]. Issues related to managing DM and approaches for the prevention of the development and progression of neuropathic complications have been actively discussed with understanding that glycemic control is an effective option for managing type 1 DM, but not type 2 DM [1, 2, 5]. In addition, there is no standard of therapy for diabetic optic neuropathy (DON) which takes into consideration the type and stage of the disease. Because DON is a manifestation of diabetic polyneuropathy (DPN), it is reasonable to correct the abnormality with the agents targeted at the pathogenetic components of DPN. The therapy for DPN includes measures aimed at achieving and maintaining stable compensation of DM, B group vitamins, and α-lipoic acid [6-13]. As retinal ganglion cell (RGC) apoptosis underlies DON, it deems reasonable to utilize neuroprotectors in the management of the disorder in an effort to stop the progression of neurodegenerative changes in RGC. The purpose of the study was to assess the clinical efficacy of combined adjunct treatment with thioctic acid, a combination of vitamins В1, В6, and В12, ethyl methyl hydroxypyridine succinate, citicoline and brimonidine tartrate in the management of advanced-stage axial DON. Materials and Methods Forty patients (63 eyes) were followed up after being diagnosed with advanced-stage axial DON based on the findings of retinal and optic nerve OCT in accordance with the clinical and diagnostic criteria [14] and classification of DON [15] that we have previously developed. Binocular optic neuropathy was found in 27 persons (54 eyes), and 13 of these were found to have degenerative-stage axial DON in the fellow eye. The adjunct group was composed of 20 patients (31 eyes) who were administered two courses a year of the following treatment as a combined adjunct adjunct to hypoglycemic therapy: (1) a 600 mg intramuscular injection of thioctic acid (Berlithion), daily for 21 days, followed by switching to oral regimen, at a dose of one 300 mg tablet twice a day for 21 days, (2) a 2 ml intramuscular injection of Milgamma, once per 3 days for 21 days, followed by switching to oral regimen, one tablet thrice a day for 21 days, (3) a 100 mg intramuscular injection of ethyl methyl hydroxypyridine succinate (Armadine), twice a day for 14 days, (4) a 500-mg intravenous bolus of citicoline (Ceraxon), twice a day for 14 days, followed by switching to oral regimen, 500 mg daily for a month. In addition, they were administered topical brimonidine tartrate 0.2%, 1-2 drops twice a day on a constant basis. The control group (20 patients; 32 eyes) received hypoglycemic therapy only. Patients underwent an examination at baseline and 1.5, 6, 7.5, 12, 13.5, 24 and 25.5 months after treatment. In addition to routine eye examination, retinal and optic nerve optical coherence tomography and electrophysiology were performed. Focal loss volume (FLV), a ganglion cell complex (GCC) parameter, was assessed as the integral of deviation in areas of significant focal GCC loss based on GCC measurements with a RTVue-100 OCT system (Optovue, Inc., Fremont, CA). Software programs LC_Thickness_programm.m and main_low_noise_filters_programm.m were used to assess lamina cribrosa thickness based on OCT measurements [16]. Electrically evoked phosphene thresholds (EPT) were measured to evaluate retinal sensitivity, and critical flicker fusion thresholds (CFFT) were assessed using electric phosphene stimulation with a KNSO-2 apparatus (FOSFEN, Odesa, Ukraine). Means (M), standard deviations (σ), standard errors of the mean (SEM) were calculated using Microsoft Excell 2000. In addition, coefficients of variation (Сν), significance (p), 95% confidence intervals (CI) were calculated. The level of significance p ≤ 0.05 was assumed. Results and Discussion No improvement, improvement to subclinical-stage axial DON, improvement to mild-stage axial DON, and progression of the disease to degenerative-stage axial DON was found in 9.7% (3 eyes), 61.3% (19 eyes), 22.6% (7 eyes) and 6.4% (2 eyes), respectively, of the adjunct group, versus no changes and progression of the disease to degenerative-stage axial DON in 40.6% (13 eyes) and 59.4% (19 eyes), respectively, of the controls at the 25.5-month time point (Fig. 1).
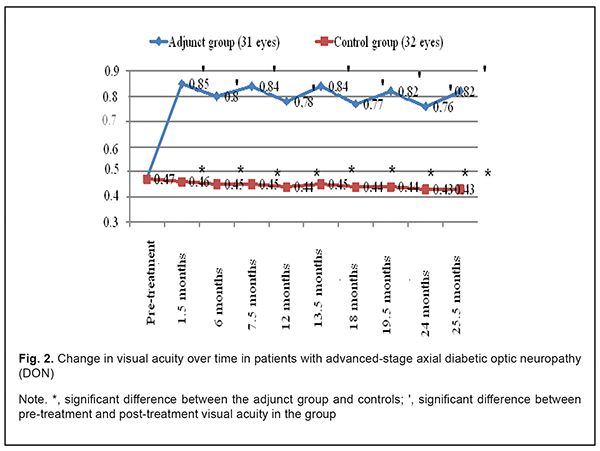
In addition, in the adjunct group, the mean visual acuity at 1.5 months, 6 months, 7.5 months, 12 months, 13.5 months, 24 months, and 25.5 months improved compared to baseline (Fig. 2). In the control group, mean visual acuity over the follow-up was not significantly different compared to baseline (0.476±0.13; р>0.05). Therefore, mean visual acuity was statistically significantly better in the adjunct group than in the control group (р<0.05) at 1.5 months, 6 months, 7.5 months, 12 months, 13.5 months, 24 months, and 25.5 months.
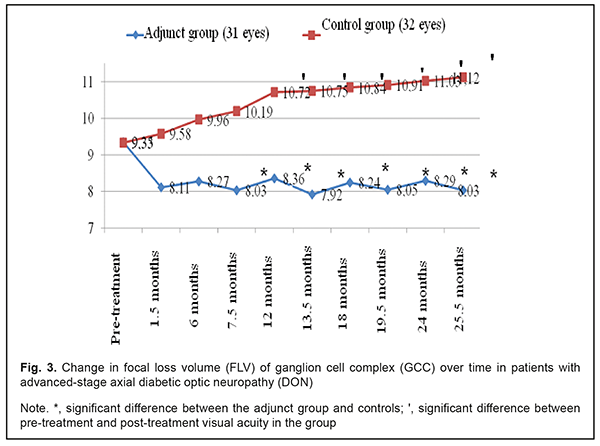
In the adjunct group, mean EPT value at 1.5 months, 6 months, 7.5 months, 12 months, 13.5 months, 24 months, and 25.5 months was 68.7±7.3 μA, 77.3±8.1 μA, 66.4±6.9 μA, 76.87±7.8 μA, 67.5±7.0 μA, 77.5±8.0 μA, and 68.2±7.5 μA, respectively, and was 55.6%, 50.0%, 57.1%, 50.4%, 56.4%, 49.9% and 55.9 %, respectively, lower compared to baseline (154.7±13.5 μA; p < 0.05). In the control group, mean EPT value at these time points was 157.9±13.0 μA, 160.2±12.7 μA, 155.9±13.1 μA, 164.0±13.9 μA, 162.8±13.4 μA, 159.7±14.5 μA, and 160.3±13.8 μA, respectively, and was not significantly different compared to baseline (154.6±13.3 μA; р > 0.05). Mean GCC FLV value in the adjunct group at 1.5 months, 6 months, 7.5 months, 12 months, 13.5 months, 24 months, and 25.5 months was not significantly different from baseline (9.35±3.39%, р > 0.05; Fig. 3). In addition, mean GCC FLV value in the adjunct group at 7.5 months, 12 months, 13.5 months, 24 months, and 25.5 months was 26.9%, 28.2%, 35.7%, 33.1%, and 38.5%, respectively, lower compared to controls, and the difference was significant (р<0.05). There were, however, no significant differences in GCC FLV between groups at 1.5 months and 6 months (p > 0.05).
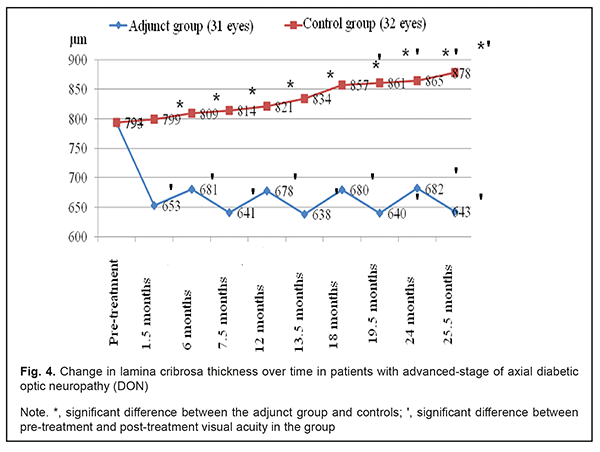
Mean thickness of the lamina cribrosa in the adjunct group at 1.5 months, 6 months, 7.5 months, 12 months, 13.5 months, 24 months, and 25.5 months was 17.7%, 14.1%, 19.2%, 14.5%, 19.5%, 14.0% and 18.9%, respectively, lower compared to baseline 793±75 μm (р < 0.05) (Fig. 4). Mean thickness of the lamina cribrosa in the control group at 1.5 months, 6 months, 7.5 months, 12 months, and 13.5 months was not statistically significantly different from baseline 794±74 μm (р > 0.05), whereas at 24 months and 25.5 months, was 865±65 μm and 878±71 μm, respectively, which was 8.9% and 10.6%, respectively (р < 0.05) greater compared to baseline. That is, compared to controls, mean thickness of the lamina cribrosa in the adjunct group at 1.5 months, 6 months, 7.5 months, 12 months, 13.5 months, 24 months, and 25.5 months was 22.4%, 18.8%, 27.0%, 21.1%, 30.7%, 26.8% and 36.5%, respectively, lower, and the difference was significant (р<0.05).
Therefore, our adjunctive treatment for advanced-stage axial DON results in improvements in study indices in 83.9% of patients, with 191% better visual acuity, 235.0% lower electrically evoked phosphene thresholds, 38.5% less GCC FLV, and 36.5% less thickness of the lamina cribrosa compared to controls at 25.5 months. Our findings are in agreement with those of the Cochrane review [1] that did not find statistically significant improvements in neuropathy markers in type II DM patients treated with intensive glycemic control. Even conventional glycemic control has failed to prevent neuropathy progression in more than 40% of patients with DM. The structural and functional characteristics of the optic nerve in our controls with advanced-stage axial DON provide evidence for feasibility of agents targeted at other pathogenetic components of DON in combination with hypoglycemic agents. Without a doubt, the use of antioxidants is reasonable, since oxidative stress is a key pathogenetic mechanism of DPN, and, that is, of DON. Alpha lipoic acid (thioctic acid) is a potent antioxidant whose clinical efficiency in the treatment of DPN has been demonstrated in several studies [6-11, 13]. Benfotiamine and pyridoxine, the two substances of Milgamma (Woerwag Pharma, Böblingen, Germany), have the ability to inhibit the formation of advanced glicated end products (AGEs) that can cause cell dysfunction, inflammatory processes and vascular wall disorders. In addition, some authors [4, 7] believe these substances to be neurotrophic inhibitors of AGEs. The accumulation of AGEs in the lamina cribrosa in DM results in collagen cross-linking and changes in the biomechanical characteristics of the plate, with an increase in its stiffness and reduction in its elasticity [17, 18]. We believe that the fact that, at 25.5 months after treatment, the thickness of the lamina cribrosa in our adjunct group was 36.5% less than in controls may be explained by the inhibitory effect of the substances of Milgamma on the accumulation of AGEs in collagen. Brimonidine tartrate is a multifactorial neuroprotective agent, and improves retinal ganglion cell survival in the presence of glutamate, oxidative stress and hypoxia [19]. The agent, however, has been found not effective in preventing neurodegeneration in diabetic patients in the absence of pre-treatment neurodegenerative changes in the GCC [20], which confirms the appropriateness of using it to treat patients diagnosed with DON. It has been reported that treatment with ethyl methyl hydroxypyridine succinate (Armadine) combined with vitamin-like substances improves mytochondrial glucose oxidation, which in turn improves ATP synthesis and neutralization of free radicals whose production is elevated under conditions of tissue ischemia [21, 22]. The use of ethyl methyl hydroxypyridine succinate with Milgamma in the comprehensive treatment for DM has contributed to an improvement in lipid peroxidation status and enhancement of tissue sensitivity to insulin [22]. Gorshkov et al [22] investigated correction of DPN symptoms, and observed a substantial reduction of neuropathic manifestations of DPN in patients treated with both ethyl methyl hydroxypyridine succinate and Milgamma. Citicoline is presently being investigated as a promising treatment option for brain ischemia, Alzheimer disease, Parkinson’s disease, non-arterial ischemic optic neuropathy, glaucoma and amblyopia [23]. Its neuroprotective effect on retinal nerve fibers under conditions of hyperglycemia has been demonstrated recently [24]. This finding is in agreement with the earlier positive findings on the effect of neurotrophin and citicoline on neuronal apoptosis and neurite regeneration in cultured rat retinas exposed to high glucose [25], and with the findings on the clinical effect of citicoline for the treatment of DPN [26], which makes the use of citicoline in DON reasonable. The findings of the current study provide evidence that the detection of DON followed by timely administration of adequate therapy attenuates the progression of disease, and may provide the basis for sight saving in a considerable body of patients with the disease. Conclusion Patients with advanced-stage axial DON were administered (1) a 600 mg intramuscular injection of thioctic acid (Berlithion), daily for 21 days, followed by switching to oral regimen, at a dose of 300 mg twice a day for 21 days, (2) a 2 ml intramuscular injection of Milgamma, once per 3 days for 21 days, followed by switching to oral regimen, one tablet thrice a day for 21 days, (3) a 100 mg intramuscular injection of ethyl methyl hydroxypyridine succinate (Armadine), twice a day for 14 days, and (4) a 500-mg intravenous bolus of citicoline (Ceraxon), twice a day for 14 days, followed by switching to oral regimen, 500 mg daily for a month, in two courses a year, with topical brimonidine tartrate 0.2%, 1-2 drops twice a day on a constant basis. This was found to result in improvements in study indices in 83.9% of patients, with 191% better visual acuity (p < 0.05), 235.0% lower electrically evoked phosphene thresholds (p < 0.05), 38.5% less GCC FLV, and 36.5% less thickness of the lamina cribrosa compared to controls at 25.5 months.
References
|