J.ophthalmol.(Ukraine).2018;5:26-31.
|
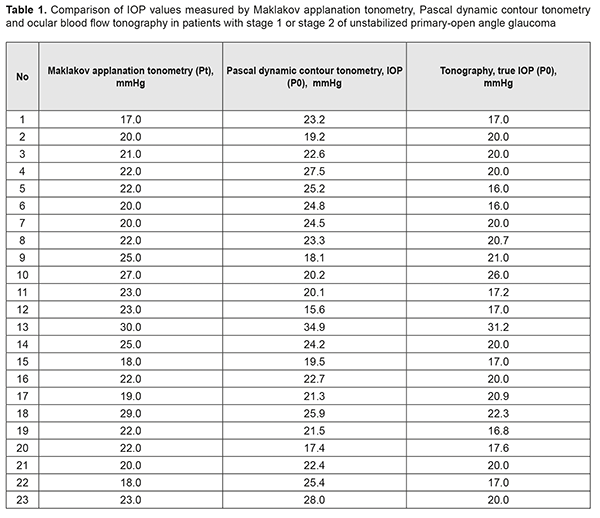
https://doi.org/10.31288/oftalmolzh201852631 Received: 23 August 2018; Published on-line: 26 October 2018 Interrelationship of corneoscleral rigidity, corneal thickness and target IOP levels in patients with unstabilized POAG O.A. Peretiagin, Cand Sc (Med), Ass. Prof.; S.K. Dmytriiev, Dr Sc (Med), Prof.; Yu. M. Lazar, Cand Sc (Med); Tatarina Yu.A., Junior Research Fellow Filatov Institute of Eye Diseases and Tissue Therapy of NAMS of Ukraine; Odessa (Ukraine) E-mail: tatarina.j.a@gmail.com TO CITE THIS ARTICLE: Peretiagin OA, Dmytriiev SK, Lazar YuM, Tatarina YuA.Interrelationship of corneoscleral rigidity, corneal thickness and target IOP levels in patients with unstabilized POAG. J.ophthalmol.(Ukraine).2018;5:26-31. https://doi.org/10.31288/oftalmolzh201852631 Background: To the best of our knowledge, no strict criteria for estimating the probability of glaucomatous progression have been reported. Purpose: To investigate the interrelationship between corneoscleral rigidity, corneal thickness, and target IOP in patients with stage 1 or 2 of unstabilized primary open-angle glaucoma (POAG). Materials and Methods: Patients underwent routine eye examination for glaucoma. In addition, Pascal dynamic contour tonometry and ocular blood flow tonography were performed, corneoscleral rigidity was determined, and target IOP was calculated. Results: Patients with unstabilized POAG were divided into two groups. Group 1 involved 9 patients (8 patients and 1 patient with stage 1 and stage 2 of POAG, respectively) whose tonometer IOP was higher than true IOP, and Group 2 involved 14 patients (1 patient and 13 patients with stage 1 and stage 2 of POAG, respectively) whose tonometer IOP was lower than true IOP. In the former group, corneoscleral rigidity values were one order of magnitude higher than those in the latter group. It is likely that the corneoscleral rigidity in eyes with stage 1 of unstabilized POAG is substantially higher than that in eyes with stage 2 of unstabilized POAG. It was noted that patients with stage 2 of POAG had low central corneal thickness of 460 μm to 515 μm. It was found that a comparatively low target IOP of 12.7 mm Hg to 13.0 mm Hg was required for patients with a rather high corneoscleral rigidity (-2.3 mm Hg, -0.7 mm Hg, 3.1 mm Hg, 6.8 mm Hg, 6.9 mm Hg), and a comparatively high target IOP of 14.2 mm Hg to 14.6 mm Hg was required for patients with lower corneoscleral rigidity (-5.5 mm Hg, -3.2 mm Hg, -1.5 mm Hg, -4.9 mm Hg, 0.8 mm Hg). Conclusion: Absolute values of target pressure required for stabilization of POAG in patients with high corneoscleral rigidity are 10.7% lower than those for patients with low corneoscleral rigidity, and this difference is statistically significant. Keywords: unstabilized POAG, primary open-angle glaucoma, corneoscleral rigidity, corneal thickness, target IOP Introduction Dr S.F. Kalfa, an outstanding ophthalmologist, believed that “the ability to decrease intraocular pressure and to maintain it within physiologic limits is essential for preventing blindness in patients with glaucoma”. As early as 1928, he demonstrated that tonometers recorded false high intraocular pressure (IOP). That is, it was difficult to determine true IOP and arrest the progression of glaucoma to blindness before the patient lost his/her visual functions. Professor Kalfa hypothesized that flattening of the cornea during applanation tonometry decreases the volume of the globe, and the aqueous displaced from the flattened portion by pressure from the tonometer increases strain in the external tunic of the eye. As the tunic is rather rigid and the globe undergoes almost no increase in volume, the intraocular fluid exerts increased pressure on the walls of the globe, i.e., IOP increases. In addition, it has been found that tonometer pressure is composed of two components, true IOP and an increase in IOP. The second component is due to (1) elastic response of ocular tunics upon contact with the tonometer, and (2) response of ocular vessels which somewhat reduces an increase in IOP during tonometry procedure [1-4]. Others conducted further research on IOP. Friedenwald analyzed the work of Professor Kalfa, and, based on this analysis and his own research on IOP measurements, introduced the notion of coefficient of rigidity in 1939. He believed that the coefficient is constant at an IOP > 5 mm Hg for middle-aged individuals [5, 6]. However, in the opinion of A.I. Dashevskiĭ, the term “coefficient of rigidity” was not completely accurate, and he introduced the notion of “coefficient of reactivity” whose value depended on several variables [7, 8]. Contrary to the opinion of Friedenwald, other writers like Dashevskiĭ (1946), and Perkins and Gloster (1957) demonstrated that the coefficient of rigidity varied with IOP. Kozlov designed a sensing mirror method for measuring elastic properties of the sclera in 1966, and demonstrated that the sclera underwent changes with IOP [9]. All these studies were among the first to employ new characteristics, corneal and scleral rigidity, in the diagnosis of glaucoma. Nesterov and Bunin proposed to consider IOP as a function of ocular rigidity [10] in 1974. Strakhov and Alekseev designed a method termed “dynamic rigidometry” as early as nineties [11]. However, the pathogenetic value of corneoscleral rigidity for the glaucomatous process is yet to be accurately determined, and some studies on the topic are underway. Besides rigidity, increased corneal thickness has received much attention as a risk factor for the onset and progression of primary open-angle glaucoma (POAG). Stewart et al [12] demonstrated that patients with thicker, mild-range and thinner cornea progressed in 14%, 18% and 32 % of cases, respectively. In addition, eyes with thinner corneas have been found to exhibit more advanced visual field changes compared with those with mid-range or thick corneas. Furthermore, an association had been found between low corneal thickness and reduced lamina cribrosa density. Although numerous studies have been performed on corneoscleral rigidity and corneal thickness in eyes with POAG, to the best of our knowledge, no strict criteria for estimating the probability of glaucomatous progression have been reported. Therefore, the purpose of the study was to investigate the interrelationship between corneoscleral rigidity, corneal thickness, and target IOP in patients with stage 1 or 2 of unstabilized POAG. Materials and Methods Medical records of patients with stages 1 (mild) or 2 (moderately advanced) of unstabilized POAG were retrospectively reviewed and analyzed. Patients underwent routine eye examination for glaucoma. In addition to Maklakov applanation tonometry (MAT), Pascal dynamic contour tonometry (PDCT) and ocular blood flow tonography (OBFT) were performed. Corneoscleral rigidity was calculated as the difference between the IOP measured by MAT and true IOP measured by PDCT [13]. In addition to kinetic Goldmann perimetry, visual fields were examined using the SITA Fast central 30-2 threshold test procedure with a Humphrey Field Analyzer (model HFA-II 750; Carl Zeiss Meditec, Inc., Dublin, CA). Moreover, optic nerve OCT was performed on spectral optical coherence tomography SOCT Copernicus Plus (Optopol, Zawiercie, Poland) to examine morphometric parameters and determine ganglion cell layer thickness. The following formula was used to calculate target IOP based on patient age and diastolic blood pressure (DBD) [14]: Р0target = 9.5 + 0.07 × DBD – 0.024 × AGE Study results were coded, entered, and analyzed using STATISTICA 7.0 и Microsoft Excel [15, 16]. Results and Discussion The study group consisted of 19 patients (23 eyes) with stages 1 (mild) or 2 (moderately advanced) of unstabilized POAG. First, we compared IOP measurements obtained by MAT, PDCT and OBFT for each study patient (Table 1).
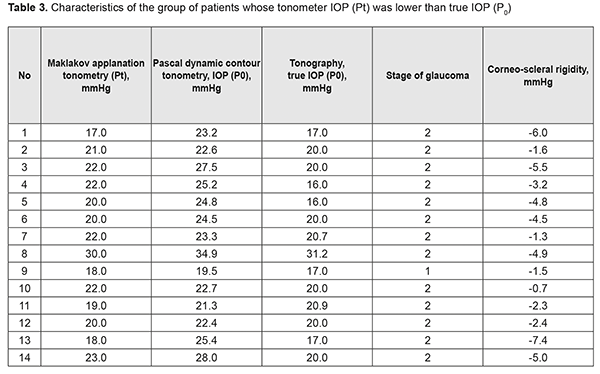
It was seen (Table 1) that for some of the eyes, the relationship between MAT IOP reading and a true PDCT IOP reading did not meet the following requirement: a true IOP reading should be a few mmHg lower than a tonometer IOP reading. Tonometer IOP (Рt) is determined by measuring the amount of force required to apply to the cornea and thus displace a constant volume of aqueous from the eye. The force influences hydrodynamics in ocular chambers, and, the more the volume displaced, the more the difference between the Pt and true IOP (P0). Patients were divided into two groups in order to analyze the results. Group 1 involved patients whose tonometer IOP was higher than true IOP (Table 2), and Group 2 involved patients whose tonometer IOP was lower than true IOP (Table 3).
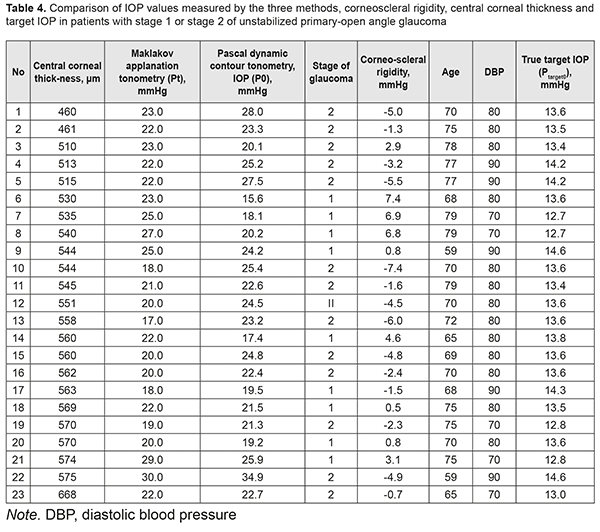
In Group 1 (9 patients), the majority (8 patients) had mild POAG, and only one patient had moderately advanced POAG, whereas in Group 2 (14 patients), the majority (13 patients) had moderately advanced POAG, and only one patient had mild POAG. Corneoscleral rigidity was determined for each eye. As this parameter was determined as a difference, expressed in mmHg, between MAT IOP reading and a true PDCT IOP reading, it was reasonable that the corneoscleral rigidity for patients of Group 1 was circa one order of magnitude higher than that for patients of Group 2 that involved patients with P0 > Рt and mostly those with moderately advanced POAG. That is why one may hypothesize that the corneoscleral rigidity for patients with stage 1 of unstabilized POAG is substantially higher than that for patients with stage 2 of unstabilized POAG. This means that corneoscleral rigidity for patients with unstabilized POAG does not depend on tonometer IOP, but does depend on the stage of glaucoma (the presence of this relationship has been already demonstrated by some authors, although with the results that were different from those obtained in the current study). For example, patient K (Table 2) with stage 1 of unstabilized POAG had the same tonometer IOP level (Pt = 22.0 mmHg) as patient H (Table 3) with stage 2 of unstabilized POAG, but P0 and corneoscleral rigidity (Ec) for the former patient were 21.5 mmHg and 0.5 mmHg, compared to 27.5 mmHg and -5.5 mmHg, respectively, for the latter patient. The studies by Svetlova and Koshits demonstrated the association between the stage of glaucoma and corneoscleral rigidity. In their opinion, the corneoscleral rigidity for eyes with glaucoma is substantially higher than that for normal eyes as early as initial stages of POAG, and there is a proportional relationship between the corneoscleral rigidity, IOP and stage of glaucoma [17, 18]. This contradicts with our findings, as we found a decrease in the corneoscleral rigidity with an increase in the stage of glaucoma. This contradiction might be explained by the fact that our patients had unstabilized POAG, whereas those in the studies by Svetlova and Koshits had stabilized POAG. Our findings are in better agreement with those of the study by Kozlov who has found that the sclera of a normal eye is much more elastic than that of the glaucomatous eye, its volumetric strain being approximately twice as that of the glaucomatous eye [19]. Therefore, one may hypothesize that the corneoscleral rigidity decreases with the onset and progression of glaucoma in eyes with unstabilized POAG. No statistically significant correlation was found through comparison of IOP and corneoscleral rigidity with central corneal thickness in all patients of the study. However, it was noted that patients with stage 2 of POAG had low central corneal thickness of 460 μm to 515 μm (Table 4). This is understandable given the well-known fact that progression of glaucoma is more common in thinner corneas. Corneal thickness has been shown previously to affect IOP readings, with thin corneas resulting in a falsely low IOP, and thick corneas resulting in a falsely high IOP. The corneoscleral rigidity varied from -7.4 mm Hg to 7.4 mm Hg in patients of the current study. In addition, as the patient age ranged from 59 years to 79 years, and diastolic blood pressure ranged from 70 mm Hg to 90 mm Hg, the true target IOP (Р0target) calculated based on these values ranged from 12.7 mm Hg to 14.6 mm Hg. Moreover, it was found that a comparatively low target IOP of 12.7 mm Hg to 13.0 mm Hg was required for patients with a rather high corneoscleral rigidity (-2.3 mm Hg, -0.7 mm Hg, 3.1 mm Hg, 6.8 mm Hg, 6.9 mm Hg), and a comparatively high target IOP of 14.2 mm Hg to 14.6 mm Hg was required for patients with a rather low corneoscleral rigidity (-5.5 mm Hg, -3.2 mm Hg, -1.5 mm Hg, -4.9 mm Hg, 0.8 mm Hg). Consequently, one might hypothesize a relationship between the corneoscleral rigidity and target IOP in patients with stage 1 or 2 of unstabilized POAG, which determines the risk of glaucomatous progression in patients with high corneoscleral rigidity.
Conclusion First, corneoscleral rigidity for patients with unstabilized POAG does not depend on tonometer IOP, but does depend on the stage of glaucoma. Second, absolute values of target IOP for patients with stage 1 or 2 of stabilized POAG are statistically significantly lower than those for patients with stage 1 or 2 of unstabilized POAG. Third, absolute values of target pressure required for stabilization of POAG in patients with high corneoscleral rigidity are 10.7% lower than those for patients with low corneoscleral rigidity, and this difference is statistically significant. Finally, an inverse correlation was found between the corneoscleral rigidity and target IOP for patients with stage 1 of 2 of unstabilized POAG.
References
|