J.ophthalmol.(Ukraine).2018;5:20-25.
|
https://doi.org/10.31288/oftalmolzh201852025 Received: 23 August 2018; Published on-line: 26 October 2018 Speed of development and speed of progression of primary open-angle glaucoma S.O. Rykov, Dr. Sc. (Med.), Prof.; A.V. Burdei, a Postgraduate; O.V. Petrenko, Dr. Sc. (Med.); S.Yu. Mogilevskyy, Dr. Sc. (Med.), Prof.; L.I. Denisyuk, Cand. Sc. (Med.) Shupik National Medical Academy of Postgraduate Education; Kyiv (Ukraine) E-mail: sergey.mogilevskyy@gmail.com TO CITE THIS ARTICLE: Rykov SO, Burdei AV, Petrenko OV, Mogilevskyy SIu, Denisyuk LI.Speed of development and speed of progression of primary open-angle glaucoma. J.ophthalmol.(Ukraine).2018;5:20-25. https://doi.org/10.31288/oftalmolzh201852025
Purpose: To perform the analysis of the results of primary open-angle glaucoma (POAG) patients’ examination at presentation and to build objective criteria for the development and progression of the disease. Materials and Methods: One hundred and seventy two patients (344 eyes) diagnosed with POAG were involved into the study. In addition, the control group comprised 98 volunteers (196 eyes) diagnosed with no POAG. The age at presentation ranged from 40 to 74 years with a mean of 57.3±1.1 years. Statistical analyses were performed using MedStat and MedCalc v.15.1 (MedCalc Software bvba). Results: Disease duration was found to increase from 0 to 3 years with an increase in the stage of POAG (Н=10869; p=0.00Е-01). Both absolute IOP values and IOP categories progressively increased with an increase in the stage of POAG (Н=88.76, р=0.00Е-01 and Н=90.13, р=0.00Е-01, respectively). Two indices, SDPOAG (speed of POAG development, expressed as stage/life year), and SPPOAG (speed of POAG progression, expressed as stage/disease year), were proposed as measures for assessing speed of the pathological process. These were found to progressively increase with an increase in the stage of the pathological process (р<0.05). The effect of SPPOAG on the stage of POAG was found to be statistically significant (F=21.1; p=2.16E-11). Keywords: primary open-angle glaucoma, speed of POAG development, speed of POAG progression
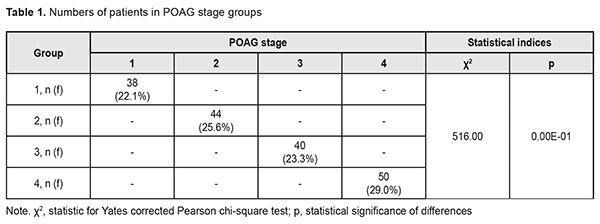
A review published in 2014 estimated the global number of primary open-angle glaucoma (POAG) cases (aged 40-80 years) in 2013 at 44 million, rising to 53 million by 2020 and 79.76 million in 2040 [1-3]. The prevalence of POAG varies between geographical regions and ethnic groups. In 2015, the number of people with the disease in Europe was estimated to be 7.81 million, increasing to 8.3 million in 2020 and 8.82 million in 2025 [4]. The prevalence rises substantially with age, from 0.1% for persons from 40 to 45 years of age to 1.5-2% for those aged 50-60 years, and 10% for the population aged 75 and older [5, 6]. Early diagnosis and proper treatment is a must to prevent POAG progression to blindness [5]. The rate of timely POAG diagnosis has been found to be 50-53% for patients followed for long periods of time (e.g, due to a concomitant ocular disorder), and substantially lower for those undergoing only screening [7, 8]. It is very important to make objective predictions for the development and progression of POAG as early as at presentation [9]. The purpose of the study was to perform the analysis of the results of POAG patients’ examination at presentation and to build objective criteria for the development and progression of the disease. Materials and Methods One hundred and seventy two patients (344 eyes) diagnosed with POAG were involved into the study. In addition, the control group comprised 98 volunteers (196 eyes) diagnosed with no POAG. The groups were matched for gender (78 (45%) men and 94 (55%) women in the POAG group versus 46 (47%) and 52 (53%), respectively, in the controls, p=0.90). In addition, the POAG group was matched for age at presentation (range, 40 to 74 years; mean age, 57.3±1.1 years; men: range, 40 to 73 years, mean age, 58.8±1.5 years; women: range, 40 to 74 years, mean age, 56.1±1.5 years; p>0.05) with the controls. Patients were divided into four groups based on glaucoma stage (classified according to Nesterov (2008) [5]) at presentation: Group 1 (stage 1 or mild POAG; no peripheral visual field changes), Group 2 (stage 2 or moderately advanced POAG; marginal excavation, arc scotoma, with a nasal visual field loss > 10 degrees or concentric visual loss ≤ 15 degrees), Group 3 (stage 3 POAG or far advanced disease with a marked visual field loss; 40 patients), and Group 4 (stage 4 or terminal-stage POAG; complete loss of vision or faulty light projection). For early glaucomatous loss (stage 1 of the Nesterov classification), moderate glaucomatous loss (stage 2 of the Nesterov classification), and advanced glaucomatous loss (stages 3 or 4 of the Nesterov classification), parametric changes were MD < -6 dB, MD < -12 dB, and MD > -12 dB, respectively. Statistical analyses were performed using MedStat and MedCalc v.15.1 (MedCalc Software bvba). Results and Discussion Distribution of patients among groups as per POAG stage at presentation is shown in Table 1.
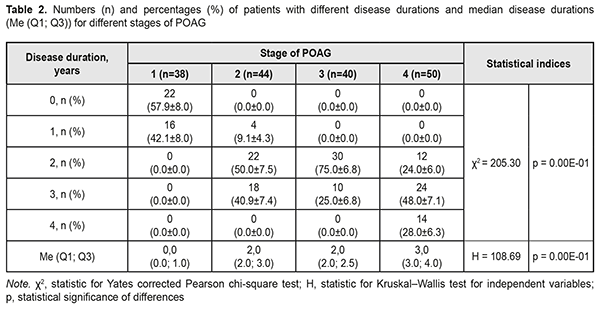
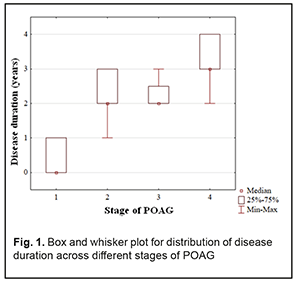
At presentation, mean disease duration for patients with stage 1, stage 2, stage 3, and stage 4 POAG was 0.4 years, 2.3 years, 2.3 years, and 3.1 years, respectively. Non-parametric methods were used to analyze data as the latter were not normally distributed. The Yates corrected Pearson chi-square test was applied to determine statistically significant differences in contingency tables of frequencies for patients with different disease durations. For independent variables, the Kruskal–Wallis test was applied to compare group medians (Table 2 and Fig. 1).
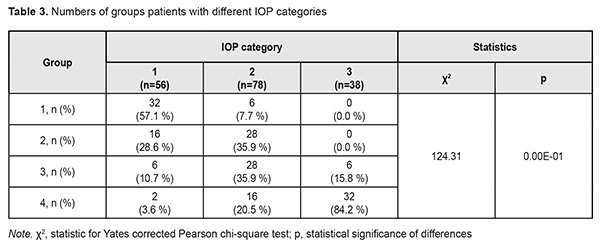
The diagnosis was made at presentation (i.e, disease duration at presentation was 0) in most (57.9±8.0%) of patients with stage 1 POAG, but in no patient with stage 2 POAG. The disease duration at presentation was either 2 years or 3 years in most (50.0±7.5 years and 40.9±7.4%, respectively) of patients with stage 2 POAG. Patients with stage 3 POAG had disease duration of either 2 years (75.0±6.8%) or 3 years (25.0±6.8%), whereas those with stage 4 POAG. Of patients with stage 4 POAG, most had disease duration of 3 years (48.0±7.1%), while others, either 2 years or 4 years. Therefore, with an increase in the stage of POAG, there was a redistribution of patients from minimum disease duration (0 years) at stage 1 to maximum disease duration (4 years) at stage 4, which was statistically significant (χ2=205.30; р=0.00Е-01). In addition, although there was no significant difference in median values between Group 2 and Group 3 (Fig. 1), there was an increase in disease duration from 0 to 3 years with an increase in the stage of POAG (Н=10869; p=0.00Е-01). IOP categories were defined as per protocol [5]: normal (category 1, up to 23 mmHg); moderately elevated (category 2, 23 mmHg to 32 mmHg); and elevated (category 3, 33 mmHg and above). Among the four POAG groups, Group 1 had the highest percentage (57.1%), and Group 4 had the lowest percentage (3.6%) of patients with category 1 IOP at presentation.
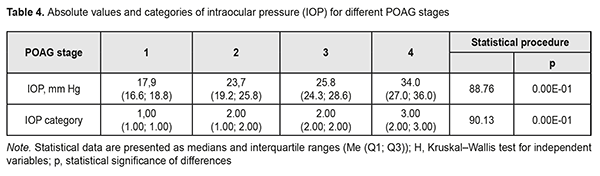
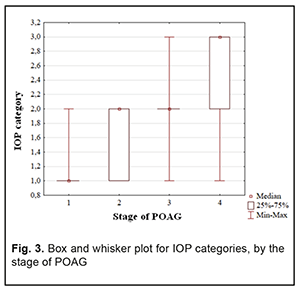
In addition, Groups 2 and 3 had equal percentages (35.9%) of patients with category 2 IOP. Group 4 had the highest percentage (84.2%) of patients with category 3 IOP, but there were no such patients in Group 1 or Group 2. Therefore, with an increase in the stage of POAG, there was a redistribution of patients from category 1 IOP at stage 1 to category 4 IOP at stage 4 (χ2=124.31; р=0.00Е-01). Both absolute IOP values and IOP categories for the groups are presented in Table 4. IOP progressively increased with an increase in stage of POAG (Н=88.76; р=0.00Е-01). The same relationship (Table 2) was observed for IOP categories (Н=90.13; р=0.00Е-01). These trends are also shown in Figures 2 and 3.
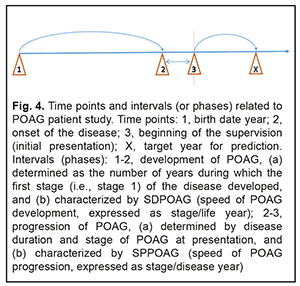
Two indices, SDPOAG (speed of POAG development, expressed as stage/life year), and SPPOAG (speed of POAG progression, expressed as stage/disease year), were used as measures for assessing speed of the pathological process. In order to determine the values of these indices, it is reasonable to consider a diagram (Fig. 4) illustrating time points and intervals relating to the study of patients with POAG.
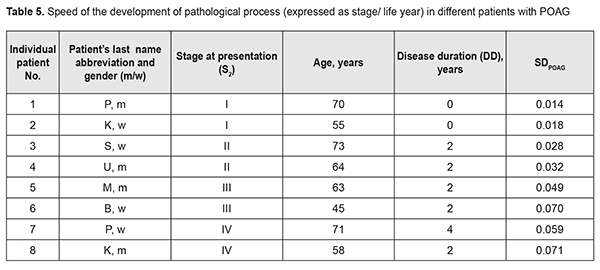
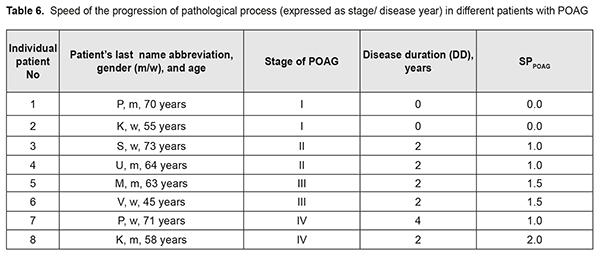
It should be noted that the onset of initial signs of POAG (time point 2) did not always coincide with the initial patient presentation at the ophthalmologist (time point 3). Therefore, the stage of POAG at time point 2 for the patient could differ from that at time point 3. At the same time, it is reasonable to believe that the onset of initial signs of POAG and the development of stage 1 POAG had to coincide in time. If the first patient’s visit occurred soon after the onset of initial signs of disease, the time difference between time points 2 and 3 was negligible, and the stage of POAG at time point 2 for the patient did not differ from that at time point 3. However, if the first patient’s visit was due to eye health impairment resulting from progression of the disease, the stage of POAG at time point 3 for the patient was likely to be more severe than that at time point 2. Based on the above considerations, we calculated the dependent variables that had been used to develop regression equations and that were used as measures for assessing speed of the pathological process as follows: SDPOAG = S2/(A – DD)(1), where SDPOAG is speed of POAG development; S2 is stage of POAG at the onset of initial signs of the disease (time point 2); A is patient’s age; and DD is disease duration at presentation (time point 3); SPPOAG = StPOAG/DD(2), where SPPOAG is speed of POAG progression; StPOAG is stage of POAG; and DD is disease duration at presentation (time point 3). Calculations of SDPOAG are exemplified in Table 5. As one can see from formula (1), SDPOAG is proportional to the stage of POAG at presentation, and inversely proportional to the difference between patient age and disease duration. Thus, the greatest SDPOAG (0.071) was found in a relatively young (58 years old) male patient K with stage 4 POAG and disease duration of 2 years.
SPPOAG characterizes the speed of disease progression with respect to the stage of disease at presentation, and is inversely proportional to the disease duration (Formula (2)). Calculations of SPPOAG are exemplified in Table 6. If the diagnosis is initially established at presentation, SPPOAG equals 0; otherwise, SPPOAG increases with an increase in the diagnosed stage of the disease. In our opinion, this allowed us to objectivize speed of the pathological process, which was important for further analysis.
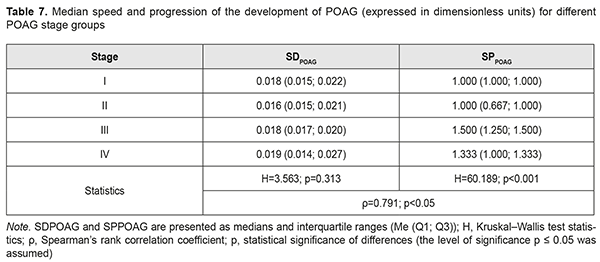
Calculations of SDPOAG and SPPOAG for POAG stage groups are presented in Table 7. In general, SDPOAG and SPPOAG increased with an increase in the stage of the pathological process, which was statistically significant (р < 0.05). In addition, a positive correlation between these characteristics pointed to a progressive parallel increase in SDPOAG and SPPOAG in patients with an increase in the stage of POAG (ρ=0.791; p<0.05). Moreover, there was no statistically significant difference in medians or spread of SDPOAG among samples (Table 7; H=3.563; p=0.313). Therefore, any POAG stage found at presentation (i.e., at time point 1 of the study) actually did not depend on the speed of the development of glaucoma. Conversely, POAG stage depended mostly on the speed of the progression of POAG (Н=60.189; р < 0.001).
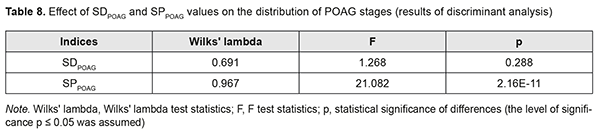
The results of the discriminant analysis of the effect of SDPOAG and SPPOAG on distribution of stages among patients with POAG are presented in Table 8, and the effect was found to be statistically significant only for SPPOAG (F=21.1; p=2.16E-11).
Conclusion First, disease duration was found to increase from 0 to 3 years with an increase in the stage of POAG (Н=10869; p=0.00Е-01). Both absolute IOP values and IOP categories progressively increased with an increase in the stage of POAG (Н=88.76, р=0.00Е-01 and Н=90.13, р=0.00Е-01, respectively). Second, two indices, SDPOAG (speed of POAG development, expressed as stage/life year), and SPPOAG (speed of POAG progression, expressed as stage/disease year), were proposed as measures for assessing speed of the pathological process. Finally, SDPOAG and SPPOAG progressively increased with an increase in the stage of the pathological process (р<0.05). The effect of SPPOAG on the stage of POAG was found to be statistically significant (F=21.1; p=2.16E-11).
References
|