J.ophthalmol.(Ukraine).2018;4:26-31.
|
https://doi.org/10.31288/oftalmolzh201842631 Received: 26 March 2018; Published on-line: 30 August 2018 Risk of developing retinopathy of prematurity in infants varying in hematological parameters, gestational age and some treatment-related factors S.V. Katsan, Cand Sc (Med), A.O. Adakhovska, Intern Physician Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine; Odessa (Ukraine) E-mail: adakhovskaya@yandex.ru TO CITE THIS ARTICLE: Katsan SV, Adakhovska AO. Risk of developing retinopathy of prematurity in infants varying in hematological parameters, gestational age and some treatment-related factors. J.ophthalmol.(Ukraine).2018;4:26-31. https://doi.org/10.31288/oftalmolzh201842631
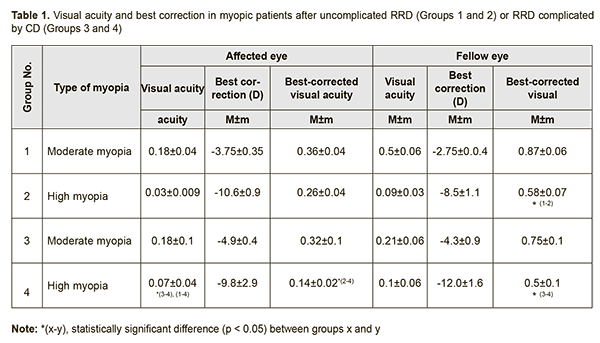
Background: Investigating risk factors for severe retinopathy of prematurity (ROP) is important, given the prevalence of ROP. Purpose: To determine the association of severe ROP with hematological parameters, gestational age and some treatment-related factors. Materials and Methods: The investigation involved a retrospective analysis of some hematological parameters (red blood cell (RBC), white blood cell (WBC) and platelet counts (PLC)) in 1248 preterm neonates. Hematological results were (a) abstracted from neonates’ medical records kept while infants were staying in neonatal care units, and (b) compared with Children’s Reference Ranges for Routine Hematology Tests at age 1 month. Statistical analyses were performed using Medstat Software version 16.8.4. Models were developed and analyzed using EZR version 1.32. Results: We found the following independent variables to be associated with an increased risk of severe ROP: low gestational age (OR, 0.52; 95% CI, 0.45 – 0.60; p < 0.001), mechanical ventilation (OR, 2.9; 95% CI, 1.4 – 5.9; p = 0.003), blood transfusion (OR, 1.23; 95% CI, 1.03 – 1.49; p = 0.02), and thrombocytopenia (OR, 0.72; 95% CI, 0.60 – 0.87; p < 0.001). Conclusion: Values of these independent variables should be taken into account when performing longitudinal screening for ROP. Keywords: retinopathy of prematurity, risk factors, thrombocytopenia, odds ratio, confidence interval Introduction Retinopathy of prematurity (RP) is a vasoproliferative eye disease that affects immature eye structures (including the retina and retinal vascular network) in preterm infants [1, 2]. Recent studies evidence that fluctuations in partial oxygen and carbon dioxide pressures in the blood and abrupt discontinuation of oxygen have a greater effect on the development of the disease than oxygen therapy [3]. Experimental studies have demonstrated that normobaric or hypobaric oxygen treatment contribute to the development of neovascularization and retinopathy in newborn animals [4, 5]. Elevated blood oxygen levels have a vasoconstrictive, obliterating and destructive effect on choroidal vessels, which decreases oxygen transport to the inner retina during hyperoxygenation of the animal. Vasoconstriction develops under conditions of hyperoxygenation, and is accompanied by tissue hypoxia during a transition to normal conditions. This, in turn, is accompanied by vasoproliferation which results from alterations in local vascular endothelial growth factor (VEGF) and systemic insulin-like growth factor 1 (IGF-1) [6]. Relative retinal hypoxia induces the expression of VEGF, a vasoproliferative factor that is required for normal development of the retinal vascular network and survival of endothelial cells. However, VEGF-stimulated vessel growth requires sufficient serum levels of IGF-1, which are deficient in premature infants due to loss of maternal sources. It is for this reason that VEGF starts accumulating in the developing retina with an increase in metabolic demand, whereas endogenous IGF-1 production begins to increase only with an increase in child’s age, which initiates VEGF activity and the development of proliferative retinopathy [7]. As platelets can accumulate and transport some major angiogenesis regulators including VEGF [8], it seems reasonable to hypothesize that they play the following role in the pathogenesis of ROP: when low blood platelet levels limit the accumulation of VEGF in blood, than, with an increased endogenous production of IGF-1, cumulative VEGF becomes activated, which leads to uncontrolled retinal neovascularization. The purpose of the study was to determine the association of severe ROP with hematological parameters, gestational age and some treatment-related factors. Materials and Methods The investigation involved a retrospective, quantitative analysis of some hematological parameters (red blood cell (RBC), white blood cell (WBC) and platelet counts) in 1248 preterm neonates (< 37 weeks of gestation with a birth weight <2500 g) who received outpatient observation care at the Filatov Institute over the period 2010 to 2016. In the current study, severe ROP was defined as a disease requiring treatment with retinal laser photocoagulation. Hematological results were abstracted from neonates’ medical records kept while infants were staying in neonatal care units of Odesa, Kherson and Mykolaiv regional hospitals and Municipal clinical hospital of Odesa. Blood samples were obtained in inpatient settings at 4 weeks postnatal. Hematological results were compared with children’s reference ranges for routine hematology tests at age 1 month (RBC, 3.1-4.3 × 1012/L; WBC, 6.0-18.0 × 109/L, and platelet count, 180-400 × 109 /L [9]). Statistical analyses were performed using Medstat Software [10]. All models were developed and analyzed using EZR version 1.32 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [11]. Incidences of independent variables are expressed as percentages and standard error percentages (±m%). The Yates-corrected chi-square test was used to compare categorical independent variables. Multivariate regression models were built to assess associations of RBC, WBC, and platelet counts with the development of type 1 ROP, threshold ROP or aggressive-posterior ROP (APROP). Predictive performance of the models was assessed for discrimination with receiver operator characteristic (ROC) curves. The area under the curve (AUC) was calculated for each ROC curve with 95% confidence intervals (CI) and its associated sensitivity and specificity were calculated. A stepwise approach with the Akaike Information Criteria (AIC) was used to select independent variables for inclusion or exclusion. Gestational age at delivery, infant’s birth weight, infant’s sex, blood transfusion, mechanical ventilation, RBC, WBC and platelet counts were considered as independent variables. Odds ratio (OR) and relevant 95% confidence interval (CI) were calculated to assess the effect of each independent variable on the risk of inefficient treatment. Results A total of 1248 records of premature neonates were included in the study. Three hundred and fifty-two infants with ROP were identified, and, of these, 133 had severe ROP (Y=1). We analyzed associations of RBC, WBC and platelet counts with the risk of the need for treatment of ROP. Multivariate regression models were developed for analyses. The overall model fit was adequate, with χ2= 20.9 and p < 0.001 for the model associated with three degrees of freedom. However, the Nagelkerke's coefficient of determination (Nagelkerke R2) was 0.043, indicating weak associations of RBC, WBC and platelet counts with the risk of the need for treatment of ROP. Table 1 demonstrates the results of analyses for each of independent variables in the three factor regression model.
Analyses revealed an increase in the risk (p<0.001) of the need for treatment of ROP with a decrease in platelet count in neonates (OR = 0.70; 95% CI, 0.60 – 0.83; for each 100 units of an increase in platelet count, with adjustment for other blood indices). It should be noted that the developed model (taking into account blood indices only) has a low predictive value. Figure 1 shows the ROC curve for the model’s predictions. The AUC was 0.59 (95% CI, 0.56 to 0.62), and was statistically significantly different from 0.5 (p < 0.001). Sensitivity and specificity of the model were 46.3% (95% CI: 36.0%–56.8%) and 80.1% (95% CI: 77.4%–82.6%), respectively.
A multivariate logistic regression model of a high predictive value was developed to determine the impact of RBC, WBC and platelet counts in conjunction with other risk factors [12] in the development of severe ROP. Table 2 presents the results of the stepwise approach that was used to select independent variables for inclusion or exclusion. Five steps of regression analysis were conducted. The final logistic regression model for predicting the risk of the need for treatment of ROP included four independent variables: gestational age, mechanical ventilation, blood transfusion and platelet count. The overall fit of the model developed with these four variables was adequate (χ2= 218.7, p<0.001 for the model associated with five degrees of freedom). The Nagelkerke's coefficient of determination (Nagelkerke R2) was 0.42, indicating strong associations of the specified variables with the risk of the need for treatment of ROP.
Table 3 shows the results of the analysis for each of the four variables used in the four-factor logistic regression model.
The risk of the need for treatment of ROP was found to decrease with each one week increase in gestational age (OR = 0.52; 95%CI, 0.45 – 0.60; p<0.001; with adjustment for potential confounders). In addition, the risk was found to increase with the use of mechanical ventilation (OR = 2.9; 95%CI, 1.4 – 5.9; p=0.003; with adjustment for potential confounders) or blood transfusion (OR = 1.23; 95%CI, 1.03 – 1.39; p=0.002; with adjustment for potential confounders). Furthermore, the risk was found to increase with each 100-unit decrease in platelet count (OR = 0.72; 95%CI, 0.60 – 0.87; p<0.001; with adjustment for potential confounders). It is noteworthy that the developed four-factor predictive model was found to have a high predictive value. Figure 2 shows the ROC curve for the model’s predictions. The AUC was 0.89 (95% CI, 0.87 to 0.93), and was statistically significantly different from 0.5 (p<0.001). Sensitivity and specificity of the model were 77.6% (95% CI: 67.9%–85.6%) and 85.0% (95% CI: 82.6%–87.2%), respectively.
Discussion Angiogenesis consists of a series of highly complex biochemical and cellular processes requiring sequential receptor activation by several growth factors, such as acidic fibroblast growth factor (FGF), basic FGF, transforming growth factor (TGF)-α, TGF-β, hepatocyte growth factor, tumor necrosis factor-α, angiogenin, interleukin (IL)-8, the angiopoietins, and vascular endothelial growth factor (VEGF) [13]. Physiological angiogenesis is required for normal vascular development, but tissue hypoxia stimulates pathological angiogenesis which causes the development of destructive processes [14, 15]. Several studies have indicated that platelets are major regulators of angiogenic regulatory proteins like VEGF [15]. It is not, however, clear whether thrombocytopenia disturbs the retinal VEGF balance and how these disturbances might the development of ROP. Vinekar et al. [16] and Jensen et al. [6] were the first to note the significant difference in platelet count between children with APROP and those without ROP. Vinekar et al. [16] described the index case that showed spontaneous resolution of APROP with numerous platelet transfusions for correcting thrombocytopenia. In the current study, we used a large sample of preterm neonates (n= 1248) to assess the association between hematological parameters and the risk of developing the disease, and found a strong association between a low platelet count and the risk of developing severe ROP. Thrombocytopenia is a major risk factor for developing ROP that requires treatment, and the degree of thrombocytopenia is inversely proportional to the severity of the course of the process. In the study by Jensen et al [6], of 91 eligible cases who underwent laser ROP surgery, 25% had thrombocytopenia, and the association was significant for zone 1 (n = 16; OR = 9.00; 95% CI, 1.14-71.0) but not for zone 2 (OR = 1.43; 95% CI, 0.54-3.75) cases and controls. Swedish researchers [16] reported that multiple infectious episodes (including sepsis, necrotizing enterocolitis, C-reactive protein ≥10 mg/L, and other clinical signs of infection that required antibiotic treatment) and thrombocytopenia particularly around the time for ROP diagnosis were associated with APROP development. The association between APROP and thrombocytopenia was statistically confirmed: all 9 APROP cases had an initial period of thrombocytopenia within the first month of life, and 6 of them had another episode of thrombocytopenia during postmenstrual age weeks 31 to 34. All APROP cases had at least two postnatal infectious episodes before the first ROP treatment. One episode occurred within the first month of life and the other occurred around the time of the first sign of ROP and/or before the APROP diagnosis. Compared to controls, APROP cases more frequently developed necrotizing enterocolitis (8/9 vs. 1/9; p<0.01) and sepsis (9/9 vs. 3/9; p<0.01). In addition, all infectious episodes in APROP cases were accompanied by thrombocytopenia (90 × 109/L, range: 4-459 vs. 158 × 109/L, range: 20-500; p<0.001). Likewise, as our investigation involved analyses of data from preterm neonates, a category of patients having numerous comorbidities, low platelet counts in these patients can in fact be associated with any of these comorbidities like sepsis, necrotizing enterocolitis, C-reactive protein ≥10 mg/L, and other clinical signs of infection that required antibiotic treatment. In addition, studies of other risk factors for ROP have been conducted. Several studies have demonstrated the effect of young gestational age and low birth weight of a premature infant on the development of ROP; moreover, the degree of retinal immaturity at birth largely determines the severity of the disease [17, 18]. A number of studies, however, found that gestational age, but not birth weight is associated with ROP. Thus, Woo and co-authors [19] found this in a cohort study including 55 consecutive twin pairs of 110 preterm infants. In a study by the EXPRESS Group [20], there was a log-linear relationship between severe ROP and gestational age at birth, with an older gestational age being associated with a lower risk of severe ROP. This agrees with our earlier finding that the risk of developing ROP decreased for each week increase in gestational age (OR=0.63, p < 0.0001). In addition, we have found that the risk of developing ROP decreased for each 100-g increase in birth weight (OR=0.63, p < 0.0001) [21]. A neonate with respiratory distress syndrome has increased requirements for extra oxygenation. A duration of oxygen support administration of ≥ 28 days results in damage to immature retinal capillaries, formation of free radicals, retinal vessel obliteration, and, finally, in revascularization. This fact has caused some researchers to hypothesize the importance of avoiding SaO2 >92-95% and fluctuations in oxygen saturation [22]. It was found that oxygen therapy duration is an independent risk factor for severe ROP that was > 40 days, > 30 days, > seven days and > five days in the studies by Niwald et al. [23], Pinheiro et al [24], and Hakeem et al [25], respectively. In a population-based cohort study by Thomas K et al [26], 1163 of 9187 (12.7%) infants developed severe ROP, and lower gestational age, male sex, patent ductus arteriosus, and more than two blood transfusions were associated with an increased risk of severe ROP. Our multifactorial analysis found that mechanical ventilation is also associated with an increased risk of severe ROP (p = 0.003; OR, 2.7; 95% CI, 1.4 – 5.2) [12]. In addition, we have reported that blood substitute transfusion was associated with an increased risk of severe ROP (p = 0.022; OR, 1.22; 95% CI, 1.03 – 1.43) [12]. Detailed knowledge of pathogenetic processes allows a better understanding of the origin of the disease, and sometimes helps in identifying etiotropic therapy that will be effective for the disease. Therefore, we found that thrombocytopenia, gestational age, mechanical ventilation, and blood transfusion were associated with the risk of the need for treatment for ROP, and in this way we determined the combination of factors that should be taken into account when performing longitudinal screening for ROP. Analysis of AUC revealed that PLC was the second most significant variable, after gestational age, associated with increased risk for severe ROP. Further research is, however, warranted to determine the efficacy of the combination of factors for predicting severe ROP. Conclusion
First, thrombocytopenia is an important risk factor for severe ROP (p < 0.001; OR, 0.70; 95% CI, 0.60 – 0.83). Second, we found that (a) gestational age, mechanical ventilation, blood transfusion, and thrombocytopenia are the variables independently associated with the risk of severe ROP, and (b) among these variables, hrombocytopenia is the second most significant, after gestational age (p < 0.001; OR, 0.72; 95% CI, 0.60 – 0.87). References
|