J.ophthalmol.(Ukraine).2018;3:32-40.
|
https://doi.org/10.31288/oftalmolzh201833240 Distribution of polymorphic genotypes of GSTP1, GSTM1 and GSTT1 and their association with primary open-angle glaucoma S.O. Rykov1, Dr Sc (Med), Prof., A.V. Burdei, Postgraduate, Ophthalmologist, S.V. Ziablitsev2, Dr Sc (Med), Prof., S.Iu. Mogilevskyy1, Dr Sc (Med), Prof. 1 Shupik National Medical Academy of Postgraduate Education; Kyiv (Ukraine) 2 Bohomolets National Medical University; Kyiv (Ukraine) E-mail: eye-bolit@ukr.net TO CITE THIS ARTICLE: Rykov SO, Burdei AV, Ziablitsev SV, Mogilevskyy SIu. Distribution of polymorphic genotypes of GSTP1, GSTM1 and GSTT1 and their association with primary open-angle glaucoma. J.ophthalmol.(Ukraine).2018;3:32-40. https://doi.org/10.31288/oftalmolzh201833240
Background: The role of genetic factors in the development of primary open-angle glaucoma (POAG) has been confirmed previously. Activation of oxidative stress with a reduction in the activity of glutathione S transferase (GST) family enzymes is a major mechanism in the pathogenesis of POAG. Since there are substantial population differences in frequencies of GST polymorphisms and there are contradictory reports regarding the association between GST polymorphisms and POAG, we considered it reasonable to investigate these polymorphisms in a Ukrainian population of patients with POAG. Purpose: To investigate the distribution of polymorphic genotypes of GSTP1, GSTM1 and GSTT1 and their associations with the development and progression of POAG. Materials and Methods: One hundred and seventy two patients (344 eyes) diagnosed with POAG were involved into the study. In addition, the control group comprised 98 volunteers (196 eyes) diagnosed with no POAG. Patients were divided into four groups based on the stages of glaucoma identified by Nesterov (2008): Group 1 (mild POAG; 38 patients), Group 2 (moderately advanced POAG; 44 patients), Group 3 (far advanced disease with markedly constricted visual fields; 40 patients), and Group 4 (terminal stage POAG, with developed blindness; 50 patients). The polymorphisms were determined using real-time polymerase chain reaction and TaqMan Mutation Detection Assays (Life Technologies). Statistical analyses were performed using MedStat and MedCalc v.15.1 (MedCalc Software bvba). Results and Discussion: We identified 11 combinations of genetic variants which were significantly different in frequency between patients with POAG and controls and between different groups of patients (χ2=112.63; p=0.00Е-01). In addition, we revealed significant differences in the distribution of genotypes between total patients with POAG and controls (χ2=54.68, p=0.00Е-01). The frequencies of the polymorphisms in the controls were different from those observed in glaucoma patients in Group 1 and Group 2 (p(χ2)=0.001 and p(χ2)=0.003, respectively), and Groups 3 and 4 (p(χ2)= 0.00Е-01). The risk for the development of stage 1 POAG was 15-fold increased in patients exhibiting the combination of GSTP1(Val/Val), GSTM1-null and GSTT1+ genotypes. The combination of GSTP1(Ile/Ile), GSTM1-null and GSTT1-null genotypes was associated with the progression of the disease, with 5.1-fold, 6.6-fold, and 13-fold increased risks for development of stage 2, stage 3 or stage 4 POAG, respectively. The combinations including the ancestral genotype GSTP1(Ile/Ile) with GSTM1 and/or GSTT1 non-null genotypes were found to be protective against progression of POAG. Conclusion: The polymorphic genotypes of GSTP1, GSTM1 and GSTT1 which result in reduced antioxidative activity are essential in both the incidence and progression of POAG. The combinations including the GSTM1 and GSTT1 mutant (null) alleles were found to be pathological, whereas those including the ancestral genotype GSTP1(Ile/Ile) with GSTM1+ and/or GSTT1 + were found to be protective against progression of POAG. Keywords: primary open-angle glaucoma, glutathione S transferase genes, GSTP1, GSTM1, GSTT1
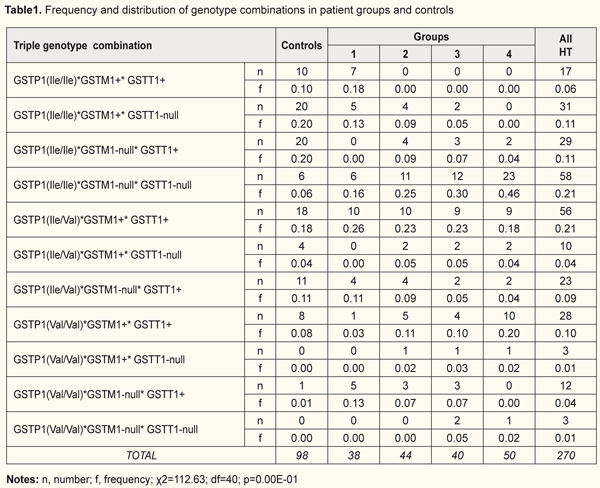
Introduction Glaucoma is characterized not only by elevated intraocular pressures, but also by the loss of retinal cells and degenerative processes in the optic nerve [1]. In addition, it is a major cause of blindness and a global and socially important issue [2]. A number of studies have confirmed the role of genetic factors in the development of the glaucoma process [3]. About 5% of POAG is currently attributed to single-gene forms of glaucoma (ie glaucoma caused by mutations mostly in myocilin) [4]. Other cases of POAG have a more complex genetic basis and are caused by the combined effects of many genetic and environmental risk factors [4-6]. Glutathione S transferase (GST) superfamily enzymes are involved in the mechanisms responsible for elimination of cytotoxic substances through (1) non-catalytic bonding of these enzymes to xenobiotics, (2) catalytic bonding of xenobiotics to glutathione, and (3) restoration of the activity of glutathione peroxidase [7, 8]. GST activity is influenced by several genes, including GSTP1, GSTM1 and GSTT1 that are located on chromosomes 11, 1, and 22, respectively. The GSTP1 gene has three allelic variants that are associated with adenine-to-guanine substitution and the consequent isoleucine-to-valine substitution at position 105, GSTP1(Ile/Ile), GSTP1(Ile/Val) and GSTP1(Val/Val) [1]. The polymorphism of GSTM1 and GSTT1 genes is associated with the absence (GSTM1+ and GSTT1+) or presence (GSTM1-null and GSTT1-null) of genomic deletions [9]. Besides the above mechanisms related to detoxification of xenobiotics, GSTP1 is also involved in cell proliferation and apoptosis mechanisms through regulation of protein kinases, particularly, c-Jun N-terminal kinases (JNKs) [8]. The change in amino acid sequence results in the synthesis of an enzyme with a reduced activity [9]. Previously, we have already demonstrated the effect of deletion GST polymorphisms on the development and progression of POAG in a Ukrainian patient population [10]. Activation of oxidative stress is a major mechanism in the pathogenesis of POAG [11], which is likely to be associated with polymorphic variants of GST genes that contribute to a reduction in the activity of GST family enzymes and development of the disease. In a disease with a multifactorial genetic basis, a combination of various polymorphisms may either neutralize or increase the pathologic effect of the genes that are associated with the development of the disease [8]. Since there are substantial population differences in inheritance of polymorphic gene variants and there are contradictory reports regarding the association between GST polymorphisms and POAG, we considered it reasonable to investigate these polymorphisms in a Ukrainian population of patients with POAG. The purpose of the study was to investigate the distribution of polymorphic genotypes of GSTP1, GSTM1 and GSTT1 and their associations with the development and progression of POAG. Materials and Methods One hundred and seventy two Kyiv Municipal Clinical Eye Hospital “Eye Microsurgery Center” patients (344 eyes) diagnosed with stage 1 to stage 4 POAG were involved into the study. In addition, the control group comprised 98 volunteers (196 eyes) diagnosed with no POAG. Patients were divided into four groups based on the stages of glaucoma identified by Nesterov (2008) and the classification of perimetry changes related to the stages of glaucoma [12]: Group 1 (mild POAG; 38 patients), Group 2 (moderately advanced POAG; 44 patients), Group 3 (far advanced disease with markedly constricted visual fields; 40 patients), and Group 4 (terminal stage POAG, with developed blindness; 50 patients). Patients underwent visual acuity testing, Humphrey perimetry, tonometry, biomicroscopy, refractometry, ophthalmoscopy, gonioscopy, corneal pachymetry and ocular coherence tomography (OCT) of the optic nerve. Real-time polymerase chain reaction (PCR) was conducted on a Gene Amp® 7500 PCR System (Applied Biosystems, Foster City, CA) with TaqMan Mutation Detection Assays (Life Technologies, Carlsbad, CA) to determine polymorphisms in the GSTM1, GSTT1, and GSTP1 genes. Fasting blood samples (0.5 ml) were drawn from the cubital vein. Statistical analyses were performed using MedStat and MedCalc v.15.1 (MedCalc Software bvba). Odd ratios (OR) and respective 95% confidence intervals (CI) were used to assess associations between alleles or genotypes and susceptibility to POAG. The level of significance p ≤ 0.05 was assumed. The statistical significance of differences between groups was evaluated with chi-squared and Fisher’s exact tests. The study was conducted in accordance with the fundamental principles of the European Convention on Human Rights and Biomedicine, the Declaration of Helsinki, and the Order of Ministry of Health of Ukraine No 690 dated 23 September 2009. Patients with POAG and controls were familiarized with the study purpose and tasks and provided written informed consent. Results and Discussion The genotypic combinations of GSTP1, GSTM1 and GSTT1 examined (Table 1). We identified 11 combinations of genetic variants which were significantly different in frequency between patients with POAG and controls and between different groups of patients (χ2=112.63; p=0.00Е-01). The GSTP1(Ile/Val)*GSTM1-null*GSTT1-null combination was detected neither in the control group nor in patients with POAG. In the control group, two triple genotype combinations, GSTP1(Ile/Ile)*GSTM1+*GSTT1-null and GSTP1(Ile/Ile)*GSTM1-null*GSTT1+, were the most commonly found (20% each), followed by GSTP1(Ile/Val)*GSTM1+*GSTT1+ (18%) and GSTP1(Ile/Val)*GSTM1-null*GSTT1+ (11%). One of the three triple genotype combinations (GSTP1(Ile/Ile)*GSTM1+* GSTT1-null, GSTP1(Ile/Ile)*GSTM1-null*GSTT1+, GSTP1(Ile/Val)*GSTM1+* GSTT1+ or GSTP1(Ile/Val)*GSTM1-null*GSTT1+) was present in the majority (two thirds) of controls. No controls were found to have the GSTP1(Val/Val)* GSTM1+*GSTT1-null or the GSTP1(Val/Val)*GSTM1-null*GSTT1-null genotype combination. The most abundant triple genotype combination in patients analyzed was GSTP1(Ile/Ile)*GSTM1-null*GSTT1-null (30%), followed by GSTP1(Ile/Val)*GSTM1+* GSTT1+ (22%) and GSTP1(Val/Val)*GSTM1+*GSTT1+ (12%). Interestingly, that the GSTP1(Ile/Val)* GSTM1+*GSTT1+ was the most common triple genotype combination in patients of Group 1 (26%), while the GSTP1(Ile/Ile)*GSTM1-null*GSTT1-null genotype was the most abundant in patients of Groups 2, 3 and 4, with the percentage increasing with an increase in severity of POAG (25%, 30% and 46%, respectively).
No patients of Group 1 were found to have GSTP1(Ile/Ile)*GSTM1-null*GSTT1+, GSTP1(Ile/Val)*GSTM1+*GSTT1-null, GSTP1(Val/Val)*GSTM1+*GSTT1-null or GSTP1(Val/Val)*GSTM1-null* GSTT1-null genotype combination. Eighteen percent of Group 1 patients had the GSTP1(Ile/Ile)*GSTM1+*GSTT1+ genotype combination, and 26% had GSTP1(Ile/Ile)*GSTM1+*GSTT1-null or GSTP1(Val/Val)*GSTM1-null*GSTT1+ genotype combinations (13% each). The GSTP1(Ile/Ile)*GSTM1-null*GSTT1-null and GSTP1(Ile/Val)*GSTM1+*GSTT1+ were the two most common genotype combinations detected in Group 2 (25% and 23%, respectively), Group 3 (30% and 23%, respectively), and Group 4 (36% and 18%, respectively). The GSTP1(Ile/Ile)*GSTM1+*GSTT1+ and GSTP1(Val/Val)*GSTM1-null*GSTT1-null genotype combinations were not detected in Group 2. No patients of Group 3 were found to have the GSTP1(Ile/Ile)*GSTM1+* GSTT1+ genotype combination. The GSTP1(Ile/Ile)* GSTM1+*GSTT1+, GSTP1(Ile/Ile)*GSTM1+*GSTT1-null and GSTP1(Val/Val)* GSTM1-null*GSTT1+ genotype combinations were not detected in any of the patients of Group 4. We revealed significant differences in the distribution of genotype combinations between total patients with POAG and controls (χ2=54.68, p=0.00Е-01). The frequencies of GSTP1, GSTM1 and GSTT1 polymorphisms in the controls were different from those observed in glaucoma patients in Group 1 (p(χ2)=0.001), Group 2 (p(χ2)=0.003), and Groups 3 and 4 (p(χ2)=0.00Е-01) (Table 2), thus providing evidence in support of the association between variable GST genotypes and development of POAG. Significant differences in distribution of genotype combinations were seen when comparing Groups 2, 3, and 4 with Group 1 (р(χ2)=0.031, (р(χ2)=0,019, and р(χ2)<0.00Е-01, respectively). However, no significant differences in distribution of genotype combinations existed between Groups 2, 3, and 4 (р(χ2)>0.09). Since patients of Group 1 had mild-stage disease, whereas those of other groups had POAG of higher severity, this might point to a potential effect of genotypes also on the progression of the disease.
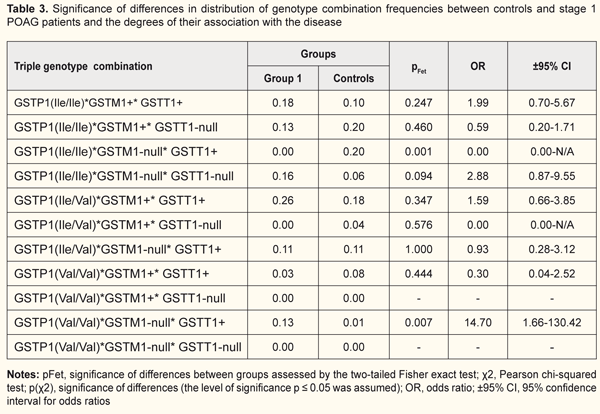
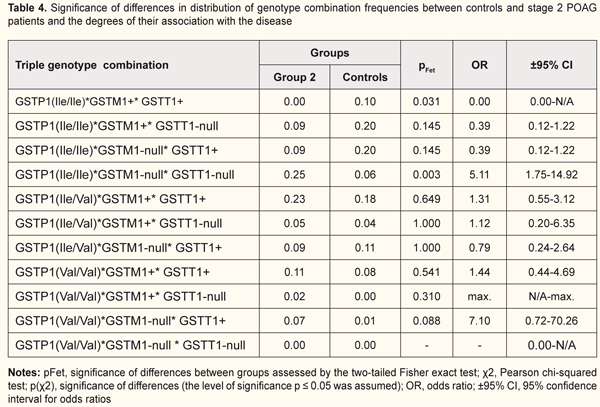
Next, we examined specific combinations of the variant genotypes of GSTP1, GSTM1 and GSTT1 for association with the disease in each group (Tables 3 to 6). The OR for the GSTP1(Val/Val)*GSTM1-null*GSTT1+ combination was 14.7 (CI: 1.66-130.42) in Group 1 patients vs controls (pFet=0.007), indicating an association of this genotype combination with mild-stage POAG (Table 3). Hence, the GSTM1-null alleles and homozygous GSTP1 Val/Val allele were found to be the polymorphisms associated with the development of POAG. Since no GSTP1(Ile/Ile)*GSTM1-null*GSTT1+ combination was found in Group 1 patients, we may hypothesize that it is the GSTP1 Val allele and not the GSTM1-null allele that is a risk allele for the development of POAG. The risk for the development of stage 1 POAG was almost 15-fold increased in patients exhibiting the GSTP1(Val/Val)*GSTM1-null*GSTT1+ combination. For the GSTP1(Ile/Ile)*GSTM1-null*GSTT1-null genotype combination, we found a statistically significant difference in frequency when patients of Group 2 were compared with controls (pFet=0.003, OR=5.11; 95% CI: 1.75 to 14.92), indicating an association of this genotype combination with POAG (Table 4). We hypothesize that it was the cumulative effect of null alleles of GSTТ1 and GSTМ1 that resulted in the development of the disease, with a significant reduction in the enzymatic activity of GST and consequent activation of oxidative stress. In addition, for those who had the GSTP1(Ile/Ile)*GSTM1-null*GSTT1-null genotype combination, the risk of developing POAG was more than five-fold increased compared with controls. No carriers of the (GSTP1(Ile/Ile)*GSTM1+*GSTT1+) combination of ancestral genotypes was found in Group 2, which provided evidence on its protective effect against progression of POAG.
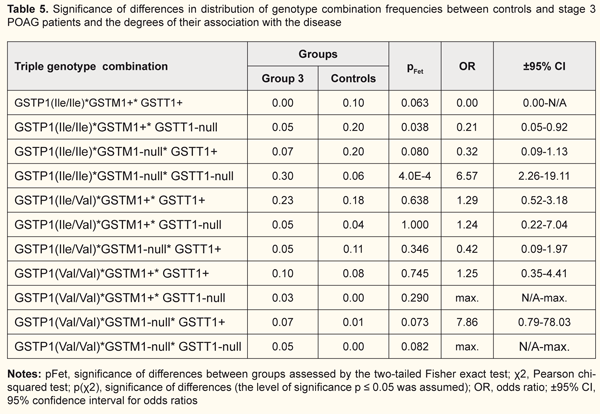
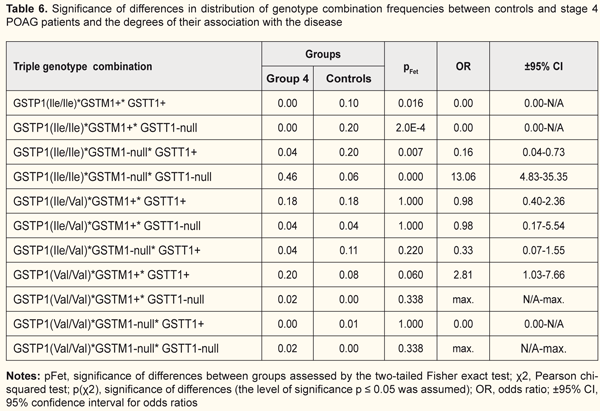
Similar data regarding an association of the pathological process with the GSTP1(Ile/Ile)*GSTM1-null*GSTT1-null genotype combination were found (Table 5) in patients of group 3 (pFet=4.0Е-4, OR=6.57; 95% CI: 2.26 to19.11). We observed that in the carriers of this combination, the progression of the disease from stage 2 to stage 3 was accompanied by an increase both in statistical significance by Fisher’s exact test and in the odds ratio (the risk of developing stage 3 POAG was increased almost 7-fold). No carriers of the GSTP1(Ile/Ile)*GSTM1+*GSTT1+ genotype combination was found also in Group 3. In addition, the frequency of the GSTP1(Ile/Ile)*GSTM1+*GSTT1-null genotype combination in Group 3 patients was 4-fold lower compared to the controls, and this difference was significant (pFet=0.038, OR=0.21; 95%CI, 0.05 to 0.92), which provided evidence on its protective effect against progression of POAG. An association of the GSTP1(Ile/Ile)*GSTM1-null*GSTT1-null genotype combination with the disease was found also in patients of group 4 (pFet=0,000, OR=13.06; 95% CI: 4.83 to 35.35) (Table 6). Therefore, for those who had the GSTP1(Ile/Ile)*GSTM1-null*GSTT1-null genotype combination, the risk of developing POAG was found to be thirteen-fold. No carriers of the protective genotype combinations (i.e., GSTP1(Ile/Ile)*GSTM1+*GSTT1+ or GSTP1(Ile/Ile)*GSTM1+*GSTT1-null) were found in Group 4 or in Group 3. Moreover, the frequency of the GSTP1(Ile/Ile)*GSTM1-null*GSTT1+ genotype combination in Group 4 patients was 5-fold lower compared to the controls, and this difference was significant (pFet=0.007, OR=0.16; 95% ВІ=0,04-0,73). Therefore, the general pattern was as follows: the percentage of carriers of the risk genotype combination (GSTP1(Ile/Ile)*GSTM1-null*GSTT1-null) increased, whereas the frequencies of the protective genotype combinations (GSTP1(Ile/Ile)*GSTM1+*GSTT1+, GSTP1(Ile/Ile)*GSTM1+* GSTT1-null or GSTP1(Ile/Ile)*GSTM1-null*GSTT1+) decreased with the progression of POAG. Since there were significant differences in frequencies of GST genotype combinations between patients of Group 1 and patients of other groups, the combinations were associated with the course of the disease. In each of Groups 2 to 4, the GSTP1(Ile/Ile)*GSTM1-null*GSTT1-null genotype combination contributed most to the progression of POAG. In addition, both the frequency of the genotype combination, and the magnitude of its association with the disease increased with the progression of POAG. Our previous works [10] have already demonstrated an association of GSTM1 and GSTT1 null (deletion) genotypes with the development and progression of POAG. Studies on genotype variability in patients with POAG may /explain some features of the course of the disease and progression of the pathological process. Activation of oxidative stress is essential in the pathogenesis of POAG. Blood rheology may become dramatically affected by active free radicals, which in turn plays a key role in the pathogenesis of glaucomatous optic neuropathy [13]. In addition, activation of oxidative stress pathway results in the changes in the trabecular wall of Schlemm canal. Lipid peroxidation products and active oxygen species can affect trabecular endothelial cells and cause abnormalities in the drainage system of the anterior eye [13]. Izzotti et al [11] have experimentally demonstrated that oxidative damage to the trabecular meshwork induces an alteration of the aqueous humor flow and triggers the “glaucomatous cascade”. Therefore, the GSTP1, GSTM1 and GSTT1 polymorphisms that result in reduced antioxidative activity are essential in both the initiation of the glaucoma process and the progression of the disease in general. Our examination of the distribution of genotypes among patients of experimental groups and controls demonstrated that the combination including null alleles of GSTM1l and GSTT1 was the most common combination associated with the disease (Fig. 1). In addition, the combinations including the ancestral genotype GSTP1(Ile/Ile) with GSTM1+ and/or GSTT1+ genotypes were found to be protective against progression of POAG. Conclusion First, we revealed significant differences in the distribution of genotypic combinations of GSTP1, GSTM1 and GSTT1 between total patients with POAG and controls (χ2=54.68, p=0.00Е-01). The frequencies of GSTP1, GSTM1 and GSTT1 polymorphisms in the controls were different from those observed in glaucoma patients in Group 1 (Fisher’s two-tailed test, p(χ2)=0.001), Group 2 (Fisher’s two-tailed test, p(χ2)=0.003), and Groups 3 and 4 (Fisher’s two-tailed test, p(χ2)= 0.00Е-01). Second, the GSTP1(Val/Val)*GSTM1-null*GSTT1+ combination was found to be a risk genotype combination for the development of mild (stage 1) POAG, and its presence was associated with an almost 15-fold increased risk for the development of mild POAG. The GSTP1(Ile/Ile)*GSTM1-null*GSTT1-null genotype combination was associated with the progression of the disease, and the association increased with increasing severity of the pathological process, with 5.1-fold, 6.6-fold, and 13-fold increased risks for development of stage 2, stage 3 or stage 4 POAG, respectively. Finally, the combinations including the ancestral genotype GSTP1(Ile/Ile) with GSTM1+ and/or GSTT1+ genotypes (GSTP1(Ile/Ile)*GSTM1+*GSTT1+, GSTP1(Ile/Ile)*GSTM1+*GSTT1-null and GSTP1(Ile/Ile)*GSTM1-null*GSTT1+)) were found to be protective against progression of POAG. References 1.Yu Y, Weng Y, Guo J, Chen G, Yao K. Association of glutathione S transferases polymphisms with glaucoma: a meta-analysis. PLoS ONE. 2013;8(1): e54037. 2.Quigley HA, Broman AT. [The number of people with glaucoma worldwide in 2010 and 2020]. Br J Ophthalmol. 2006 Mar;90(3):262-7. 3.Kirilenko MYu, Churnosov MI. [Genetic research of primary open-angle glaucoma]. Bulletin of Tambov University. Series: Natural and Technical Sciences. 2014;19(4):1140-2. Russian. 4.Fingert JH. Primary open-angle glaucoma genes. Eye (Lond). 2011;25(5);587-95. 5.Mogilevskyy SIu, Ziablitsev SV, Denisiuk LI. [Gender and age-related features of the association between TP53 Pro72Arg polymorphism and primary open-angle glaucoma]. Journal of Ophthalmology (Ukraine). 2016;4:15-19. Ukrainian 6.Mogilevskyy SIu, Ziablitsev SV, Denisiuk LI, Gur’ianov VG. [Mathematical analysis of the effect of Pro72Arg polymorphism in the TP53 gene on the emergence and progression of POAG]. Journal of Ophthalmology (Ukraine). 2016;6:32-7. Ukrainian 7.Kolesov SA, Rakhmanov RS, Blinova ТV, Strakhova LA. [New data on the diagnostic potential of cytosolic glutathione S-transferases]. Int J Applied and Basic Research. 2016;3:577-80. Russian. 8.Teplyakov AT, Berezikova EN, Shilov SN. [Heart failure: Clinical and genetic aspects of ischemic remodeling and myocardial apoptosis in the development of heart failure]. Tomsk: SST. 2015. Russian. 9.Wang W, Zhou M,, Chen S, Zhang X. Association of glutathione S-transferase polymorphisms (GSTM1 and GSTT1) with primary open-angle glaucoma: an evidence-based meta-analysis. Gene. 2013;526(2):80-6. 10.Rykov SA, Burdei AV. [Association of deletion polymorphisms of the glutathione-S-transferase gene in patients with primary open-angle glaucoma]. Archive of Ophthalmology of Ukraine. 2017;5;3(9):61-7. Ukrainian. 11.Izzotti А, SaccaS.С., Longobardi M, Cartiglia C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Invest Ophthalmol Vis Sci. 2009 Nov;50(11):5251-8. 12.Nesterov AP. [Glaucoma]. Moscow: Meditsinskoye Informatsionnoye Agenstvo; 2008 Russian. 13.Erichev VP, Egorov EA. [On the pathogenesis of primary open-angle glaucoma]. Vestn Oftalmol. 2014 Nov-Dec;130(6):98-104. Russian.
|