J.ophthalmol.(Ukraine).2018;3:27-31.
|
https://doi.org/10.31288/oftalmolzh201832731 Changes in corneal scleral rigidity and corneal thickness at various target intraocular pressures in patients with stabilized primary open-angle glaucoma Peretyagin O.A., Cand. Med. Sc., Ass. Prof.; Dmitriev S.K., Dr. Med. Sc., Prof.; Lazar Yu.M., Cand. Med. Sc.; Tatarina Yu.A., Junior Research Fellow SI “Filatov Institute of Eye Diseases and Tissue Therapy of NAMS of Ukraine”; Odessa (Ukraine) E-mail: tatarina.j.a@gmail.com TO CITE THIS ARTICLE: Peretyagin OA, Dmitriev SK, Lazar YuM, Tatarina YuA. Changes in corneal scleral rigidity and corneal thickness at various target intraocular pressures in patients with stabilized primary open-angle glaucoma. J.ophthalmol.(Ukraine).2018;3:27-31. https://doi.org/10.31288/oftalmolzh201832731
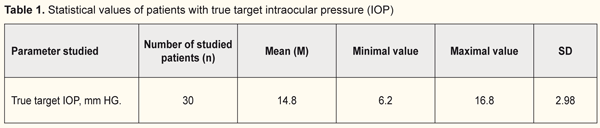
Introduction. Current methods for determining target IOP have disadvantages. Investigation of data on corneoscleral rigidity and corneal thickness in patients with stabilized primary open-angle glaucoma of stages I-II may improve the method of determining target IOP. Purpose. To study the correlation between indices of corneoscleral rigidity, corneal thickness and target IOP in patients with stabilized primary open-angle glaucoma (POAG) of stages I-II. Material and Methods. 30 patients (30 eyes) with stabilized POAG of the І-ІІ stage were examined. The patients underwent pachymetry, applanation tonometry, dynamic contour tonometry, corneal rigidity determination and tonography. The progression of glaucoma was monitored by the HFA II Central 30-2 Threshold Test and SOCT Copernicus + units. Target IOP was calculated taking into account the age and the level of diastolic blood pressure. Results. It was found that, in group 1 with target IOP of 6.2 -13.9 mm Hg, the corneal thickness was 540-590 μm and rigidity of the cornea ranged from 1.0 to 4.0 mm Hg; while in group 2 with target IOP of 14.0 -16.8 mm Hg, the corneal thickness was 510-570 μm and rigidity of the cornea ranged from -3.0 to 1.5 mm Hg. Conclusions. It was found that the lower a value of achieved target IOP, the higher corneal rigidity in patients with stabilized stage I-II POAG. Keywords: target intraocular pressure, primary open-angle glaucoma, corneal thickness, corneal rigidity
Introduction A key point for effective treatment of patients with primary open-angle glaucoma (POAG) is reducing intraocular pressure (IOP) to target IOP. Target IOP is an upper limit of IOP at which it is possible to control damage to inner eyeball structures and breakdown of visual function [1]. The existing methods for determining target IOP suffer from a number of shortcomings since it is determined empirically considering all risk factors of a patient: baseline IOP, a glaucoma stage, a progression rate during follow-up, age, and diastolic blood pressure [2]. This keeps out of accurate determining a target IOP level. Since there is no generally recognized method to determine tolerant and target IOP, observational studies on stabilization of glaucomatous process in optimal POAG treatment are running. In the literature, there are single data on a role of corneoscleral rigidity in the development of glaucoma [3]. To-date, corneascleral rigidity is considered as corneal stiffness that characterizes an ability of fibrous tunica of the eye to resist changing the shape under external and internal macroactions [4]. It has been found that rigidity of the fibrous tunica of the eye is pathologically increased even at the early stage of glaucoma; herewith, rigidity progresses gradually, reflecting the stages of glaucoma, with a sudden change only in going from the advanced to end stage of glaucoma [5]. It should be noted that a clinical value of such a parameter as corneascleral rigidity has not been determined completely in glaucoma diagnostics and it is important to detail its common sense and prognostic value for patients with POAG. Unlike cornealscleral rigidity, the corneal thickness is a more investigated parameter which has an effect on IOP and glaucoma diagnostics in general. Previously, corrective mechanisms have been developed for calculating an IOP level in dependence on the central corneal thickness (CCT). Analyzing CCT values and corresponding IOPs has shown that with a decrease in the central corneal thickness there is a decrease in intraocular pressure according to applanation tonometry readings [6]. Later on, J.H. Liu has demonstrated that tonometry errors mostly depend on the corneal biomechanics rather than on the corneal thickness [7]. We suppose that studying values of corneal rigidity and thickness in patients with stabilized primary open-angle glaucoma of stages I-II can contribute to improving the method for determining target IOP. Purpose. To study the relationship between values of corneascleral rigidity, corneal thickness, and target IOP in patients with stabilized primary open-angle glaucoma of stages I-II. Material and Methods 20 patients (30 eyes) with stabilized primary open-angle glaucoma of stages I-II were examined. All patients were performed a comprehensive ophthalmic examination including visual acuity testing using the Sivtsev-Golovin tables, autokeratorefractometry, perimetry, biomicroscopy, ophthalmoscopy, pachymetry, applanation tonometry by Maklakov, dynamic contour tonometry, corneal rigidity testing, computed corneal topography, optical A-scanning, tonography A vision field analyzer (HFA II Central 30-2 Threshold Test) and SOCT Copernicus were used to monitor changes in glaucomatous and morphological parameters. Maklakov tonometry and dynamic contour tonometry were performed. Maklakov applanation tonometry was made by a 5g tonometer. True IOP was determined by computed tonography. Dynamic contour tonometry was performed using a PASCAL tonometer which operates on a dynamic contour based on a physical phenomenon of the Pascal law. A tonometer head, contacting with the cornea, is of a concave shape with a contour duplicating a curve of the anterior corneal surface and enables to minimize the impact of corneal properties on readings. The piezoresistive pressure sensor is integrated into the contour. The contour has a 10.4 mm radius or 32.5D according to keratometry, which makes it possible to use for the cornea a device with a curve radius over 5-6 mm (55-65D) and central thickness of 300-700 µm. In such conditions, the curvatures of the cornea and the contour match in a certain area with minimal pressure on the eyeball (less than 1 g) and the sensor registers IOP by ‘a direct transcorneal method’ [8]. Rigidity of the corneoscleral coat of the eye was determined as a difference between IOP measured by Maklakov tonometry and true IOP measured by PASCAL tonometry [9]. Target IOP was calculated in consideration with age and diastolic blood pressure (DBP) using a formula: Р0 target = 9.5 + 0.07 × DBP – 0.024 × age [2]. Statistical data were processed by methods recommended for medical research [10, 11]. All data obtained were based into a specially designed form, in which they were analyzed and simultaneously coded into digital values as a numerical, ordinal, and nominal scale. Database creation, statistical and graphical analyses were made on a personal computer using licensed software, including application software packages STATISTICA 7.0 and Microsoft Excel. When analyzing the statistical data, we determined sample-based parameters which are given in tables and have meanings as follow: М – mean; m –error of mean; n – a number of patients in the studied group; SD – standard deviation of mean, р – a level of significance. The differences were accepted significant with a zero hypothesis probability less than 5% (р <0.05). Results and Discussion Target IOP, recommended due to age and DBP, was achieved in all 30 patients (30 eyes) with stabilized POAG of stages II-III and was within the range between 6.2 and 16.8 mm Hg (Table 1). True IOP was assessed using tonography. Stabilization of glaucomatous process was controlled considering the parameters of mean deviation (MD), pattern standard deviation (PSD) on computed perimetry; parameters of optic disc cupping, a cup-to-disc ratio, cupping area, a vertical cup-to-disc ratio; and OCT data on the mean thickness of retinal nerve fibers in all quadrants in dynamics. Glaucomatous process stabilization control showed that the patients in the studied group had a stabilized form of stage I-II glaucoma.
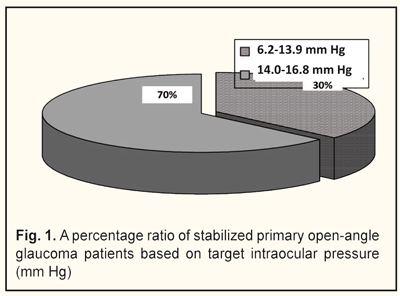
Based on a level of target IOP, we divided the patients into two groups: group 1 comprised 11 patients with target IOP ranging from 6.2 to 13.9 mm Hg; group 2 comprised 19 patients with target IOP ranging from 14.0 to 16.8 mm Hg (Fig. 1).
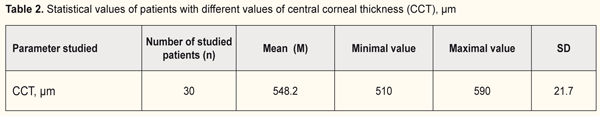
The central corneal thickness was measured in all patients and its range was from 510 to 590 μm (Table 2).
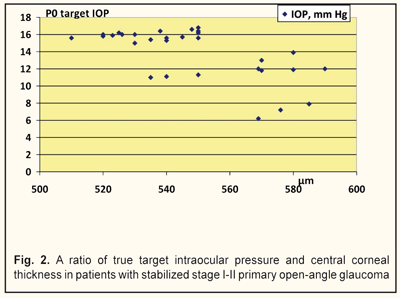
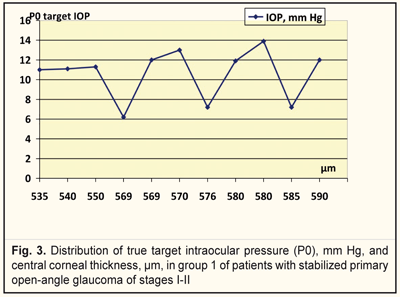
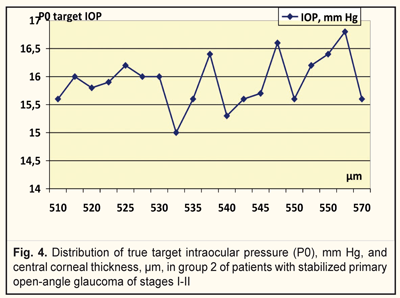
We analyzed, evaluated, and compared the OCT data of the studied groups with different target IOPs (Fig. 2). Thus, the corneal thickness in group 1 (target IOP = 6.2 -13.9 mm Hg) was equal to 540 to 590 μm (Fig. 3) while in group 2 (target IOP = 14.0 -16.8 mm Hg) that ranged from 510 to 570 μm (Fig. 4).
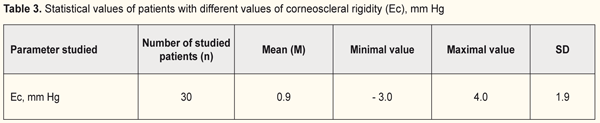
Afterwards, corneoscleral rigidity was calculated in the patients of each group (Table 3).
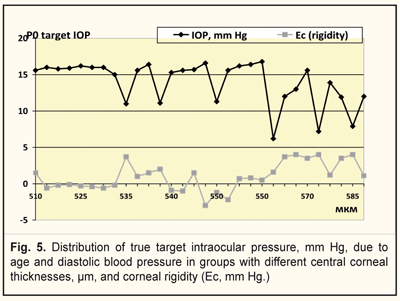
It was found that rigidity of the cornea ranged from 1.0 to 4.0 mm Hg and from -3.0 to 1.5 mm Hg in group 1(with a target IOP of 6.2 -13.9 mm Hg and corneal thickness of 540-590) and 2 (with a target IOP of 14.0 -16.8 mm Hg and corneal thickness of 510-570), respectively (Fig. 5). Figure 5 demonstrates that the lower a value of achieved target IOP, the higher corneal rigidity in the patients with stabilized stage I-II POAG.
Conclusions First, our findings point at the fact that a value of target IOP must be lower in patients with higher rigidity than in patients with low rigidity. Second, when calculating and controlling a level of target IOP in POAG patients, values of the corneal thickness and corneoscleral rigidity should be considered. References 1.Vodovozov AM. [Tolerant and intolerant intraocular pressure in glaucoma]. Volgograd; 1991. 160p. In Russian. 2.Fokin VP, Balanin SV. [Determination of target intraocular pressure in patients with primary open-angle glaucoma] Glaukoma. 2007;6(4):16-9. In Russian. 3.Boriskina LN, Balanin SV, Makovkin EM. [Corneoscleral rigidity as a cumulative biometric parameter]. Vestnik OGU. 4(153):48-50. In Russian. 4.Svetlova OV, Koshits IN. [Physiological functions of fibrous tunic of eye and their operative mechanisms. Normal and pathological physiology of eye. Textbook]. SPb: Izdatelstvo GBOU VPO SZGMU im. I.I. Mechnikova; 2013. 71p. In Russian. 5.Svetlova OV. [Functional characteristics of interaction of the sclera, accommodation and drainage systems of the eye in glaucomatous and myopic pathology]. Author’s thesis for Dr. Med. Sc. Moscow; 2010:24-7. In Russian. 6.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophtalmol. (Copenh). 1975;53:34-43. 7.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J. Cataract. Refract. Surg. 2006;32(7):1073-6. 8.Kaufmann C, Bachmann LM, Thiel MA. Comparison of dynamic contour tonometry with Goldmann applanation tonometry. Invest Ophthalmol Vis Sci. 2004;45:318-21. 9.Gontijo L. Corneal rigidity in numbers http://escrs.conference2web.com/content/4496// 27th Congress of the ESCRS : – Barcelona, – 2009. 10.Glantz S. [Medical and biological statistics. Translated from English by Danilov YuA]. M.:Praktika;1999.459p. In Russian. 11.Lapach SN, Chubenko AV, Babich PN. [Statistical methods in medical and biological investigations using Excel]. K.:Morion LTD; 2000. 320p. In Russian.
|