J.ophthalmol.(Ukraine).2018;3:22-26.
|
https://doi.org/10.31288/oftalmolzh201832226 State of reduced potential of glutathione and lipid peroxidation in tear of extended wear soft contact lens wearers T.A. Veliksar, a PhD student; M.F. Leus, Dr. of Med. Sc., Prof.; T.B. Gaydamaka, Dr. Med. Sc.; I.M. Mikheitseva, Dr. of Biol. Sc.; S.G. Kolomiichuk, a research fellow SI “The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine”; Odessa (Ukraine) E-mail: tveliksar@gmail.com TO CITE THIS ARTICLE: Veliksar TA, Leus MF, Gaydamaka TB, Mikheitseva IM, Kolomiichuk SG. State of reduced potential of glutathione and lipid peroxidation in tear of extended wear soft contact lens wearers. J.ophthalmol.(Ukraine).2018;3:22-26. https://doi.org/10.31288/oftalmolzh201832226 Background. Contact lens wearing is connected with microtraumatism of the corneal epithelium and the presence of hypoxia in anterior eye tissues. Purpose. To study values of the oxidation-reduction potential and markers of lipid peroxidation in the tear of extended wear soft contact lens wearers. Material and Methods. Patients were divided into two groups: Study Group, 20 patients (40 eyes) with mild/moderate myopia with extended soft contact lens wear; Control Group, 13 patients (24 eyes) with mild/moderate myopia, spectacles wearers. Results. A level of reduced glutathione in Study Group was by 18.3% lower than that in Control Group (p <0.05). A level of oxidized glutathione in Study Group was increased by 21%compared to Control Group (p <0.05). A level of malondialdehyde in Study Group was increased by 27.1 % compared to Control Group (p <0.05). A level of diene conjugates was icreased in Study Group, comprising 118% compared to controls (p> 0.05). Conclusions. Extended soft contact lens wear disrupts the prooxidant-antioxidant balance in the anterior eye tissues, particularly in the cornea. There is activation of free radical processes and reduction of antioxidant reserves, which is expressed in the increase of normal content of lipid peroxidation products in the tear and violation of glutathione balance. Such metabolic changes in the eye require an antioxidant therapy. Key-words: myopia, soft contact lens, cornea, glutathione, lipid peroxidation, malondialdehyde, diene conjugates
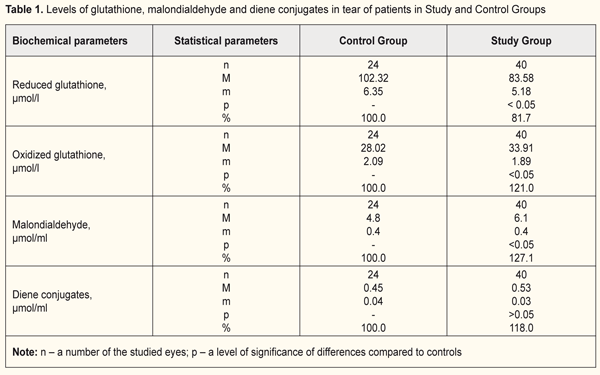
Background A contact lens is a barrier for normal connection of the anterior corneal surface with air, which is associated with a risk of acute and chronic hypoxia and, consequently, acidosis of tissues [9, 23]. Contact lens wearing is connected with microtraumatism of the corneal epithelium and the presence of hypoxia in anterior eye tissues, which results in inducing the release of cytokines, growth factors, and other inflammatory mediators [16]. Chronic hypoxia, especially in extended wearing contact lenses, is characterized by dysfunction of the epithelium which leads to violation in epithelial hydration and causes contact lens intolerance. The presence of chronic hypoxia in the anterior cornea induces adaptive mechanisms which can be divided in two phases. The first phase is a stage of emergency adaptive response and the second one is a stage of steady long-term adaptation. The cornea switches to a higher energy-consuming level of cellular metabolism. However, with prolonged influence of an unfavorable factor there is a depletion of cell energy resources, which contributes to cell apoptosis [6]. Jalbert I., Stapleton F., Efron N. et. al. have shown that extended contact lens wear reduces keratocyte density in the human cornea due to dysgenesis or apoptosis of keratocytes [12,15,16]. A number of investigators have revealed in tear of contact lens wearers a significant increase in a level of nitrous oxide which supports cell apoptosis in anterior eye tissues [8]. Studies on tear fluid composition in patients and experimental animals in keratitis and other conditions have made it possible to determine a significant relationship between the dynamics of the indices and the character of clinical manifestations of the disease [3]. It is known that tear is “an indicator” of metabolic disorders in eye pathology and reflexes the state of eye tissues, particularly of the cornea [2, 10, 13, 14, 23]. The tear fluid contains different compounds: antioxidant complexes, glutathione, vitamin C, superoxide dismutase, lactoferrin and others which protect the corneal epithelium from the effects of negative factors [18]. Glutathione is a major component of the thiol group, a state of which, as an indicator of the presence of oxidative stress, has been studied in various eye diseases [17, 21, 20]. The protective role of glutathione lies in detoxicative, anti-oxidative, and membrane-stabilizing functions, antiviral action, and regulation of inflammatory and immune processes [11, 19, 22]. Experimental studies have revealed a significant increase in reduction potential of thiol compounds in animals with keratitis and dry eye syndrome. A decreased level of reduced glutathione in the cornea occurs mainly due to accelerated glutathione oxidation under the influence of oxidative stress, which causes an increase in the permeability of the membrane structures of corneal epithelium cells [3]. Previously, studying a quantity of intracellular enzymes in tear, we have pointed at the fact that the lability of cells and cellular structures is increased in contact lens wear [1]. Since tissue hypoxia occurs in contact lens wear, the role of lipid peroxidation (LPO) in the destruction of cell membranes is highly probable. Purpose. To study values of the oxidation-reduction potential and markers of lipid peroxidation in the tear of extended wear soft contact lens wearers. Material and Methods The study involved 33 patients (64 eyes) with mild/moderate myopia. The patients were divided into two groups. Study Group (SG) comprised 20 patients (40 eyes) with mild/moderate myopia who had worn contact lens for extended period of time. Control Group (CG) comprised 13 patients (24 eyes) with mild/moderate myopia who were spectacles wearers. Of 20 SG patients, there were 13 (65%) women and 7 (35%) men. The average age was 27.45 (± SD 1.43) years, ranging from 17 to 49. The contact lens wear duration averaged 6.6 years (± SD 5.5), beginning from 1 to 22 years. Uncorrected visual acuity (UCVA) was 0.122 (± SD 0.020), ranging from 0.01 to 0.5; best corrected visual acuity (BCVA) ranged from 0.1 to 1.0 and averaged 0.845 (± SD 0.026). All patients had no subjective complaints and applied for contact lens replacement. All the patients used soft contact lenses referring to the first group according to FDA classification: non-ionic soft contact lenses with low water (<50%) content. Of 13 CG patients, there were 10 (76.9%) women and 3 (23.1%) men. The average age was 27.69 (± SD 2.0) years, ranging from 20 to 48. UCVA was 0.265 (± SD 0.139), ranging from 0.01 to 0.8; BCVA ranged from 0.4 to 1.0 and averaged 0.963 (± SD 0.197). Tear of the studied patients was collected on filter paper and placed into an Eppendorf tube. In the tear fluid we studied a glutathione level (oxidized and reduced forms) and lipid peroxidation products (malondialdehyde and diene conjugates). A method to determine a level of reduced glutathione is based on a reaction between glutathione and methylglyoxal in the presence of the glyoxalase enzyme resulting in the formation of S-lactoyl-glutathione conjugate which has maximum absorption at a 240 nm wavelength. A SF-26 spectophotometer with a wavelength of 240 nm was used for the measurements [4]. A method to determine oxidized glutathione is based on the fact that the enzymatic reduction of glutathione by glutathione reductase results in oxidation of a reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) (NADPHN2), the decreased quantity of which can be recorded spectrophotometrically at a wave length of 340 nm. A range of the determined levels of reduced and oxidized forms was 5 to 200 µg/ml in the studied solution. Mean values of a coefficient of variation for determing glutathione in the specified range was 4.0% and 5.0% for the reduced and oxidized forms, respectively. The SF-26 spectophotometer with an "optical density” working range with the optimal interval between 0.1-0.5 was used for the measurements. The content of glutathione was presented in μmol/l [4]. A method to determine a malondialdehyde level is based on the fact that malondialdehyde reacts with 2-thiobarbituric acid at the temperature of 100°С in acid medium, thus creating a stained trimethine complex with maximum absorption at a wavelength of 532 nm. Optical density of the upper phase was measured using a Specol – 210 spectrocolorimeter with a wavelength of 535 nm against butanol. Method’s coefficient of variation was 5.2% [4]. A method to determine a diene conjugate level is based on the appearance of the system of conjugated double bonds in lipid peroxidation at the stage of a free radical formation in molecules of polyunsaturated higher fatty acids, which is accompanied by appearance of new maximum in the absorption spectrum of 233 nm. Optical density of the studied solution was measured using the SF-26 spectophotometer with a 233 nm waveleangth against ethyl alcohol. The level of diene conjugates was estimated considering the molar extinction coefficient, 2.2 • 105 M-1 • sm-1, and presented in μmol / ml [7]. Data obtained were analyzed using statistical software with a SPSS 11.0 package. Statistical significance of differences was defined using the Student t-test. Results and Discussion Data on levels of glutathione, malondialdehyde and diene conjugates in tear of the patients in Study and Control Groups are given in Table 1.
Glutathione is one of the important components of the antioxidative system in the body. It occurs in oxidized and reduced forms. Misbalance of glutathione forms decreases reductive and protective properties of the system, which can lead to increased oxidation of and, consequently, damage to protein molecules in tissues. A reduced glutathione level in the SG patients (extended wear SCL wearers) was (83.58 ± 5.18) μmol/L, which was significantly lower by 8.3% compared to Control Group. A level of oxidized glutathione was higher by 21% in Study Group compared to controls (p<0.05). In view of the role of glutathione in detoxification processes and in cornea’s resistance to various pathological effects, misbalance in this system can be considered to cause a decrease in protective and adaptive potential of the cornea and other anterior eye structures in extended wearing soft contact lenses. Studying a level of lipid peroxidation products in the tear fluid showed increased accumulation of LPO products in Study Group compared to controls. However, a degree of manifestation and statistical significance of changes in primary and secondary LPO products in the tear fluid was different in the patients. Thus, a level of secondary LPO products of malondialdehyde in Study Group was significantly increased, at average, to (6.1 ± 0.4) μmol/ml, which comprised 127.1% compared to Control Group, (4.8 ± 0.4) μmol/ml (p<0.05). Levels of primary LPO products of diene conjugates in the tear fluid in Study Group was increased and comprised 118 % compared to controls, (0.53 ± 0.03) μmol/ml vs. (0.45 ± 0.04) μmol/ml, respectively; however, the changes were not statistically significant (p>0.05). This can be associated with rapid oxidation in the tear of diene conjugates to secondary LPO products. Our findings on pathochemical alterations in the level of metabolites in the tear fluid under contact lens correction conditions can be related to increased lability of cell membrane components and subcellular organelles. These alterations can be caused by insufficient oxygen supply to cornea tissues and, consequently, formation of underoxidized metabolism products such as aldehydes, acids, etc. It is commonly known that LPO processes are stimulated under such conditions, which can cause damage to lipid components of membrane structures. Indeed, our studies showed that the concentration of end LPO products was increased (of malondialdehyde – by 27.1%) in the tear fluid of the contact lens wearers. In addition, levels of primary LPO products in the tear also tended to be increased. Conclusions Our findings showed that extended soft contact lens wear disrupts the prooxidant-antioxidant balance in the anterior eye tissues, particularly in the cornea. There is activation of free radical processes and reduction of antioxidant reserves, which is expressed in the increase of normal content of lipid peroxidation products in the tear and violation of glutathione balance. Thus, we determined the decreased reduced glutathione levels and the increased oxidized glutathione levels in the tear. These changes are characteristics for a decreased reductive status of glutathione as well as for decreased antioxidant potential in the anterior eye. In parallel with these changes in eyes of extended wear soft contact lens wearers, we found accumulations of LPO products of malondialdehyde and diene conjugates in the tear fluid, which testifies to activation of LPO processes in cellular membranes and subcellular organelles in the structures of the anterior eye. Such metabolic changes in the eye require correction. So, our findings speak for the necessity and relevance of an antioxidant therapy. References 1.Veliksar TA, Leus NF, Gaydamaka TB, Mikheitseva IN, Drozhzhyna GI, Kolomiichuk SG. [Effect of Silicone Hydrogel Contact Lenses on Stability of Cellular and Intracellular Membranes in Corneal Epithelium]. Oftalmol Zh. 2017;6:7-10 2.Volkov VO, Moshetova LK. [A contemporary view of tear fluid, its importance in diagnostics]. Russkii meditsinskii zhurnal. 2004;4:138-40. Russian. 3.Gaydamaka TB, Rafalyuk SYa. [Influence of endotoxin-induced keratitis on the reducing potential of glutathione in the cornea of animals with experimental syndrome of the dry eye]. Oftalmol Zh. 2014;6:72-7. Russian 4.[New methods of biochemical analysis]. Izd. Leningradskogo univer; 1991. 395 p. Russian. 5.Terekhina NA, Petrovich YuA, Batuieva RA, Borovik GA, Reuk SE. [Diagnostic and prognostic value of the determination of the activity of tear enzymes in viral eye lesions]. [Abstracts of symposium with international participation “non-invasive methods of investigations]. Moscow;1994:42-3. Russian. 6.Fedorov AA, Egorova GB, Bobrovskikh NV. [Influence of long-term wearing of contact lenses on the state of the cornea according to confocal microscopy]. Vestn oftalmol. 2008;6:25-9. Russian. 7.Bergmeyer HU. Methoden der enzymatischen Analyse. Herausgegeben von H. U. Bergmeyer. Berlin;1986:2198-203. 8.Bhatia RP, Shikha Dhawan, Khanna HD, Amitabh DO. Indirect evaluation of corneal apoptosis in contact lens wearers by estimation of nitric oxide and antioxidant enzymes in tears. J. Ophthalmol. 2010;3(2):66-9. doi: 10.4103/0974-620X.64229 9.Cavanagh HD, Petroll M, Alizadeh H et al. Clinical and diagnostic use of in vivo confocal microscopy in patients with corneal disease. Ophthalmology. 1993;100(10):1444-53. 10.Crouch RK, Goletz P, Snyder A, Coles WH. Antioxidant enzymes in human tears. J. Ocul. Pharmacol. 1991;7(3):253-8. 11.Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 2002;64:1019-26. 12.Efron N, Perez-Gomez I, Morgan PB. Confocal microscopic observations of stromal keratocytes during extended contact lens wear. Clin. Exp. Optom. 2002;85:156-60. 13.Ichijima H, Ohashi J, Cavanagh HD. Effect of contact-lens-induced hypoxia on lactate dehydrogenase activity and isozyme in rabbit cornea. Cornea. 1992;11(2):108-13. 14.Iskeleli G, Karakoc Y, Akdeniz-Kayhan B et al. Comparison of tear lactate dehydrogenase activities of different types of contact lens wearers and normal control group. CLAO J. 1999;25(2):101-4. 15.Jalbert I, Stapleton F. Effect of lens wear on corneal stroma: Preliminary finding. Aust. NZJ Ophthalmol. 1999;27:211-3. 16.Kallinikos P, Efron N. On the etiology of keratocyte loss during contact lens wear. Invest. Ophthalmol. Vis. Sci. 2004;45:3011-20. 17.Marchitti SA, Chen Y, Thompson DC, Vasiliou V. Ultraviolet radiation: cellular antioxidant response and the role of ocular aldehyde dehydrogenase enzymes. Eye Contact Lens. 2011;37(4):206-13. doi: 10.1097/ICL.0b013e3182212642. 18.Pastori V, Tavazzi S, Lecchi M. Lactoferrin-loaded contact lenses: eye protection against oxidative stress. Cornea. 2015;34(6):693-7. doi: 10.1097/ICO.0000000000000435. 19.Pauly A, Meloni M, Brignole-Baudouin F. Multiple endpoint analysis of the 3D-reconstituted corneal epithelium after treatment with benzalkonium chloride: early detection of toxic damage. Invest. Ophthalmol. Vis. Sci. 2009;50:1644-52. 20.Saijyothi AV, Fowjana J, Madhumathi S et al. Tear fluid small molecular antioxidants profiling shows lowered glutathione in keratoconus. Exp. Eye Res. 2012;103:41-6. doi: 10.1016/j.exer.2012.07.010. 21.Varma SD, Kovtun S, Hegde KR. Role of ultraviolet irradiation and oxidative stress in cataract formation-medical prevention by nutritional antioxidants and metabolic agonists. Eye Contact Lens. 2011;37(4):233-45. doi: 10.1097/ICL.0b013e31821ec4f2. 22.Wu G, Fanf Y-Z, Yang S. Glutathione metabolism and its implication for health. J. Nutrition. 2004;134:489-92.
23.Ziadi M, Moiroux P, d'Athis P, Bron A, Brun JM, Creuzot-Garcher C. Assessment of induced corneal hypoxia in diabetic patients. Cornea. 2002;21(5):453-7.
|