J.ophthalmol.(Ukraine).2018;3:10-16.
|
https://doi.org/10.31288/oftalmolzh201831016 Outcomes of accelerated corneal collagen cross-linking in keratoconus Drozhzhyna G.I., Dr. Med. Sc., Prof.; Troychenko L.F., Cand. Med.Sc.; Naumenko V.A., Dr. Med. Sc., Prof.; Ivanova O.N., Cand. Med.Sc.; Sereda E.V. SI “The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine”; Odessa (Ukraine) E-mail: cornea@te.net.ua TO CITE THIS ARTICLE: Drozhzhyna GI, Troychenko LF, Naumenko VA, Ivanova ON, Sereda EV. Outcomes of accelerated corneal collagen cross-linking in keratoconus. J.ophthalmol.(Ukraine).2018;3:10-16. https://doi.org/10.31288/oftalmolzh201831016
Keratoconus (KC) is a degenerative noninflammatory disease of the cornea which is characterized be structural changes in the cornea which leads to progressive thinning and bulging in the central and/or peripheral part of the cornea and is accompanied by irregular astigmatism and significant vision impairment. Today, keratoconus treatment is focused on a stage of disease development. At progressive KC stages (II-III), corneal collagen cross-linking is performed. At the present, a latest-generation unit (UV-X™2000, Avedro) is used to perform accelerated cross-linking, which allows for a threefold decrease of the procedure duration (to 10 minutes) compared to the standard protocol Material and Methods. Corneal collagen cross-linking was performed in 71 patients (100 eyes). Results. Accelerated corneal collagen cross-linking for keratoconus stages II-III made it possible to stabilize the pathological process in 100% of cases, based on 12 month follow-up. Keratoconus stabilization was accompanied by: a decrease of astigmatism by 1.4 D and of the corneal refractive power by 3.2D; an increase of the corneal thickness by 8.8 nm; an increase of UCVA and BCVA in 96.5% and 89.6% of cases, respectively; and recovery of normal corneal architectonics according to confocal biomicroscopy data. Subjectively, 64 patients (90%) noted the improvement in the vision quality and better tolerance to spectacles correction. Key-words: keratoconus, corneal collagen, accelerated cross-linking
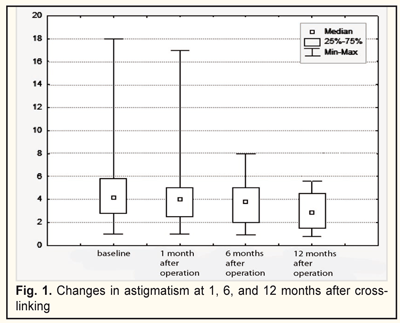
Keratoconus (KC) is a degenerative noninflammatory disease of the cornea which is characterized by structural changes leading to progressive thinning and bulging in the central and/or peripheral part of the cornea, and is accompanied by irregular astigmatism and significant vision impairment. In general population, KC affects 50-200 per 100 000 people [1]. KC is most commonly diagnosed in adolescence with both eyes affected [7, 14]. Worldwide, an age range of KC incidence is increasing; it ranges from 10 to 89 y/o and prevails at the age of 21-37, at an average [4, 5]. About 20% of keratoconus patients require corneal transplantation. In Ukraine, keratoconus is the second most frequent indication for keratoplasty [7, 14]. Etiology of the disease is still unclear. Most scientists tend to the inherited-metabolic mechanism of keratoconus development with the enzyme defect. There is evidence speaking for a genetic nature of keratoconus, including a bilateral character of affection as well as a frequent combination with inherited diseases and syndromes (blue sclera, Leber's amaurosis, Ehlers-Danlos disease, Marfan syndrome, Down syndrome, pigmentary retinopathy, aniridia, microcornea) [2,4,13]. Constant ultraviolet irradiation of the cornea and radiation can induce KC development. The presence of autoimmune processes in the body (bronchial asthma, eczema, allergic rhinitis, atopic dermatitis, and other immune disorders), long-term corticosteroid intake, affected internal secretion glands, disordered metabolism, and frequent stresses can cause KC development. Wearing incorrectly fitted contact lenses and a habit to rub eyes with hands induce trauma of keratocytes and indirectly increase the hydrostatic pressure on the eye, which leads to stretching the cornea in a cone-like shape. Being in dust rooms, where dust particles induce corneal microtrauma, also contributes to KC development. Some eye diseases such as keratoconjunctivitis and various keratitides also can cause the development of keratoconus [6, 10]. In keratoconus, all corneal layers are affected. Epithelial cells are enlarged in size and stretched. Degenerative changes in basal epithelial cells are caused by damage to the basement membrane of the epithelium. The stroma contacts with the corneal epithelium in sites of dissolution of Bowman's layer [6]. Alterations in Bowman's layer and anterior stromal layers are common and accompanied by collagen fragmentation and a fibroblastic activity. As the disease progresses, the corneal stroma gets thinned; the architectonics of collagen fibrils is destroyed with fibrils decreased in quantity and fiber diameter and expanded intefibrillar spaces. Keratocytes have membranous anomalies; cellular pleomorphism and polymegethism appear in the endothelium. With an increase in severity and duration of the disease there are changes in Descemet's membrane such as breaks and detachment [20]. All above changes result in the stretching and bulging of the cornea since the biomechanical stability of the cornea is reduced [3, 20]. Studying the topography of the cornea is the most sensitive method today for diagnosing both early and advanced keratoconus. Criteria for keratoconus progression are: failure of spectacle and contact lens correction; intolerance to old glasses; rapid vision loss (over 1 D a year); and the increased curvature of the cornea. Other signs are: corneal stroma loss with opacity on the cone top; the presence of the Kayser-Fleischer ring; biomicroscopically revealed Vogt's striae; changes in the corneal curvature revealed by corneal topography (corneal topography values > 45 D, corneal thickness < 500 μm) [1,4]. Today, keratoconus treatment is focused on a stage of disease development. The most common KC classification is one by Amsler (1998), as well as by Puchkovskaia-Titarenko (1982). At early KC stages (I-II), spectacles and contact lens correction is used to correct astigmatism. At progressive KC stages (II-III), corneal collagen cross-linking is performed and corneal ring segments are inserted. At advanced KC stages (IV-V), keratoplasty is performed [6, 16, 18, 20]. At the World Ophthalmology Congress (Vienne, Austria) in 2011, corneal ultra violet-crosslinking (CXL) was officially approved as a gold standard for early keratoconus treatment. Illumination with UV-A light activates a photochemical reaction of ionization and dissipation of riboflavin molecules with release of free atomical oxygen. Free oxygen-derived radicals cause cross-linking of -СН and –CN groups in collagen molecules, which induces their bounding in a sole 3D-mesh. Numerous additional bounds between corneal collagen fibers result in a significant improvement of corneal mechanical strength and rigidity. Biomechanical studies have shown that corneal rigidity increases by 350%-380% after cross-linking [6, 9, 13, 18, 22]. Main changes in cross-linking occur within anterior outer stromal layers (300 µm) while deeper layers remain nearly intact. Indications for cross-linking are progressive keratoconus of stages II-III with the corneal thickness of not less than 400 nm. Contradictions for cross-linking are viral eye diseases, pathological alterations of the cornea (opacities, scarring, et. al.), concomitant eye infections, autoimmune diseases, severe dry eye syndrome [6, 8]. There are several protocols for a cross-linking procedure: the original cross-linking procedure with irradiance of 3 mW/cm² which is performed according to the 'Dresden protocol' for 30 minutes; the accelerated CXL procedure with irradiance of 9.0 mW/cm² and 18 mW/cm² which is performed for 10 and 5 minutes, respectively; transepithelial CXL (without corneal epithelial debridement) [6, 21, 22]. The advanced accelerated CXL procedure with rapid recovery of the corneal architectonics takes advantages of the combined action of riboflavin (Vitamin B2) and UVA light. It has taken standard cross-linking to a new level since the time of corneal irradiation is reduced from an hour to 3 minutes [6, 15]. The accelerated CXL procedure is now possible due to the increased UVA power and the reduced exposure time with the eye-exposing energy preserved the same as in the standard procedure. At the present, a latest-generation unit (UV-X™2000, Avedro) is used to perform accelerated cross-linking, which allows for a threefold decrease of the procedure duration (to 10 minutes) compared to the standard protocol [6]. In this regard, the purpose of our investigation was to assess the outcomes of accelerated corneal collagen cross-linking in keratoconus of stages II-III. Material and Methods Corneal collagen cross-linking was performed in 71 patients (100 eyes), 45 men and 26 women aged from 12 to 57 (25.3±8.55 SD, mediana: 25). Of them, KC was diagnosed by Amsler’s classification as follows: II stage KC, 46 eyes (46%); III stage KC, 54 eyes (54%). II and III KC stages were distributed evenly among the men and women (46% and 54%, respectively). The disease duration before the CXL procedure ranged between 1 to 20 years (3±2.65 SD). In 55.1%, KC was diagnosed within 2 years. In the men, KC was diagnosed significantly more frequently at terms up to 5 years while in the women KC was commonly diagnosed within 2 years (x²=28.3, р=0.0004). Taking history showed than refraction errors (myopia, astigmatism) were observed in the family in 46% of the men; 44.6 patients noted progressive KC after stress. In the women, refraction errors in the family were noted in 53.8%; KC progression was noted post partum in 53.8% and after stress in 19.2%. In addition to standard ophthalmological examination the patients were performed biomicroscopy, refractometry, best corrected and uncorrected visual acuity, and confocal biomicroscopy of the cornea. Corneal topography, pachymetry, corneal refractive power measurements were performed using an OCULUS Pentacam® unit (Germany). Changes in the refractive power and thickness of the cornea were assessed according to a criterion of Kmax and a thinnest local criterion, respectively. Uncorrected and best corrected vision acuity was tested using the Shevalev-Sivtsev tables and assessed as three categories as follows: 1 category-VA≤ 0.1; 2 category-VA between 0.12 and 0.5; 3 category-VA between 0.55 to 1. Confocal biomicroscopy was performed using the ‘Confoscan 4’ unit (Nidek, Japan) [8]. All examinations were performed before the procedure, at 1, 6, and 12 months. The UV-X™2000 unit with irradiance of 9 mW/cm² was used to perform a CXL procedure. Corneal epithelial debridement was conducted with diameters of 7.0, 7.5, and 8.0 mm according to corneal topography data. Intraoperatively, we performed control corneal pachymetry: once after epithelial debridement and after 15 minute riboflavin saturation of the cornea. The CXL procedure itself lasted 10 minutes followed by a bandage contact lens application. In the postoperative period, antiseptics, cornea-regenerative drugs, preservative-free tear substitutes, antibiotics, and antiviral drugs were prescribed due to medical necessity. The bandage contact lens was removed from the ocular surface when complete epithelialization of the corneal surface was achieved. Statistical data included calculations of descriptive characteristics: arithmetic mean (М) and geometric mean (Мg); error of mean (m), median (Me), quartile (Q), standard deviation (SD), range of variation (Lim). Normal distribution was assessed using the Shapiro-Wilk test and the Kolmogorov-Smirnov test. For pair-wise comparison we used the Student's test with the Levene test for compulsory testing homogeneity of variance or the non-parametric Mann-Whitney U-test. The one-way ANOVA test (F-criterion) or the Friedman test (x² index) were used to compare indices measured in three or more conditions using the same sample. Correlation between variables was assessed using the Kendall concordance test. Statistical calculations were processed using the Statistica 9.0 software package. Results A follow-up period of keratoconus patients after a CXL procedure was 1 to 12 months. 100 eyes were operated on; of them, 69 and 29 eyes were followed-up for 6 and 12 months, respectively. The corneal surface was epithelialized within 3-5 days (with an average of 4.0±0.7SD days). Corneal epithelialization was noted at Day 3, 4, and 5 in 26 (26%), 48 (48%), and 26 (26%) eyes, respectively. Consequently, the bandage contact lenses were removed. No intraoperative complications occurred in all patients. Astigmatism was 4.3±2.0SD, 3.3±1.6SD, and 2.9±1.5SD (x²=74.3; р=0.000) at baseline (before CXL), at 6 and 12 months, respectively, which is demonstrated in Figure 1.
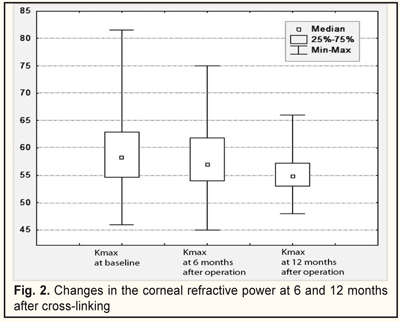
The baseline refractive power of the cornea (Kmax) ranged between 45.9 and 81.6 D (58.6±6.7SD, mediana 58.3). After CXL, it significantly decreased by 1.2D (57.4±6.7SD; mediana, 57.0) and by 3.2D (55.4±6.7SD; mediana, 54.8), (x²=54.1; р=0.02) at 6 and 12 months, respectively (Fig. 2).
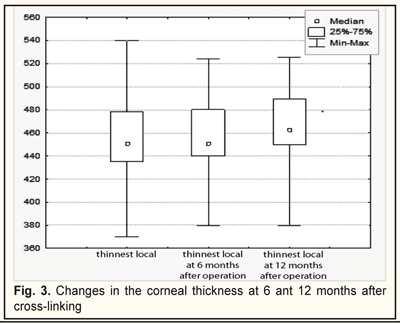
The thinnest local corneal thickness was 455.5±32.6 SD nm (mediana, 451.0) at baseline; at 6 months the cornea was not thinned and equaled 455.8±30.3 SD nm (mediana 451,0). At 12 months, the corneal thickness significantly increased to 464.3±31.2 SD nm (mediana, 462.5), (x²=34.2; р=0.000) (Fig.3).
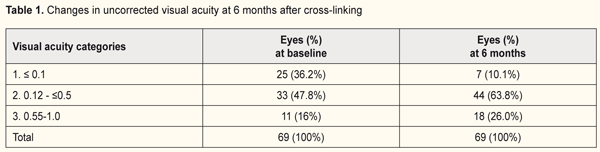
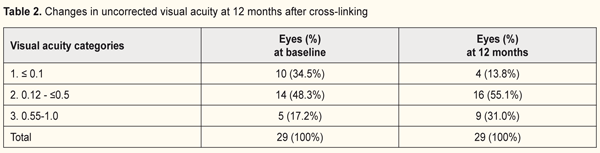
Changes in uncorrected visual acuity (UCVA) after CXL at 6 and 12 months are demonstrated in Tables 1 and 2.
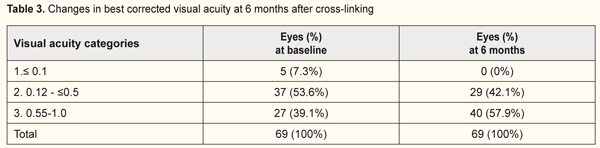
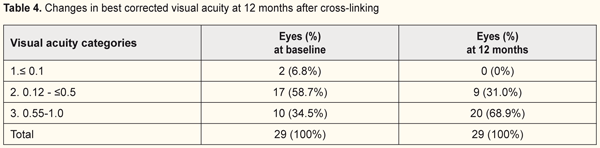
The quantity of eyes with VA<0.1 reduced from 37% to 13.8% while the percentage of eyes with high vision acuity increased after treatment at 6 months of follow-up (from 48% to 55.1% and from 15% to 31% for categories 2 and 3, respectively). In general, UCVA increased in 68 of 69 eyes (98%) at 6 months and in 28 of 29 eyes (96.5%) at 12 months (р=0.000). Best corrected visual acuity (BCVA) ≤0.1 was noted in 8 eyes (8%) at baseline; BCVA was >0.1 in all patients at 6 and 12 months after treatment. The percentage of eyes with high vision acuity was found increased at 6 and 12 months after surgery: from 39.1% to 57.9% and from 34.5% to 68.9%, respectively. (Tables 3 and 4).
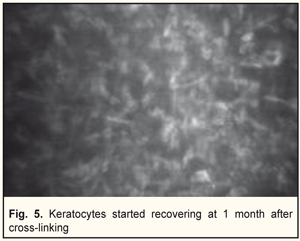
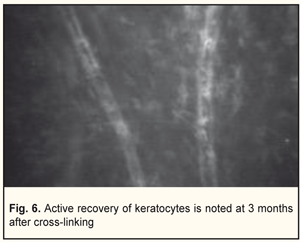
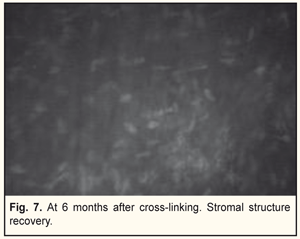
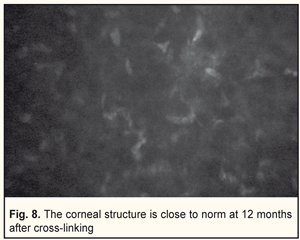
In general, BCVA increased in 62 of 69 eyes (89.8%) at 6 months and in 26 of 29 eyes (89.6%) at 12 months, which was statistically significant, р=0.000. Confocal biomicroscopy of the cornea of the patients with keratoconus after CXL revealed: at 7 days after surgery there was extracellular matrix transparency reduction, a lot of active keratocytes, collagen fibers and hypocellularity foci, i.e signs of keratocyte apoptosis (Fig. 4). At 1 month after surgery, keratocytes in the stromal layers started recovering, which was expressed in regression of the above symptoms; there still were noted hypocellularity foci and numerous active keratocytes, single Langerhans cells (Fig. 5). At 3 months after the CXL procedure, we observed an active recovery process in keratocytes in deep and superficial stromal layers with resolution of fibrosis foci (Fig. 6). At 6 months, the stromal structure was recovered; stromal folds could be seen with limited focal low hyper-reflectivity in the site of CXL (Fig. 7). At 12 months after the CXL procedure, the corneal structure was close to norm with single active keratocytes and a week focal area of hyper-reflectivity (Fig. 8).
In the post-operative period, there were sterile infiltrates in three eyes. The infiltrates were reabsorbed at days 7-10 with pathological process control and corneal epithelialization. Thus, accelerated corneal collagen cross-linking for stages II-III keratoconus made it possible to stabilize the pathological process in 100% of cases, based on 12 month follow-up. Keratoconus stabilization was accompanied by: a decrease of astigmatism by 1.4 D and of the corneal refractive power by 3.2D; an increase of the corneal thickness by 8.8 nm; an increase of UCVA and BCVA in 96.5% and 89.6% of cases, respectively; and recovery of normal corneal architectonics according to confocal biomicroscopy data. Subjectively, 64 patients (90%) noted the improvement in the vision quality and better tolerance to spectacles correction. Conclusions A procedure of accelerated corneal collagen CXL using a UV-X™2000 unit makes it possible, in addition to keratoconus stabilization, to improve vision acuity, to reduce astigmatism, and to recover the normal corneal architectonics within a 12 month follow-up period. References 1.Bikbov M.M., Surkova V.K. Corneal collagen crosslinking for keratoconus. A review. Ophthalmology in Russia. 2014;11(3):13-19. https://doi.org/10.18008/1816-5095-2014-3-13-19 2.Birich T.A., Chekina A.Yu., Aksenova N.I. [Results of treatment in patients with keratoconus]. Oftalmologiia Belarusi. 2010;1(4). In Russian. 3.Ivanovskaia EV, Vit VV, Golovchenko VG. [Immune status in patients with various stages of keratoconus and keratoglobus]. Oftalmol Zh. 2000;5:40-4. In Russian. 4.Sevastiyanov EN, Gorskova EN, Ekgard VF. [Keratoconus (etiology, pathogenesis, medicinal treatment): textbook]. Chelyabinsk: UGMADO;2005.32p. In Russian. 5.Solodkova EG, Remesnikov IA. [Modern approaches in the treatment progressive keratectasia]. Prakticheskaia meditsina. 2012;4:75-9. In Russian. 6.Adel Alhayek, Pei-Rong Lu. Corneal collagen crosslinking in keratoconus and other eye disease. Int J Ophthalmol. 2015; 8(2): 407–418. doi: 10.3980/j.issn.2222-3959.2015.02.35 7.Croghale NS. Epidemiology of keratoconus. Indian J. Ophthalmol. 2013;61(8): 382-3. 8.Kymionis GD, Portaliou DM, Bouzoukis DI, Suh LH, Pallikaris AI, Markomanolakis M, Yoo SH. Herpetic keratitis with iritis after corneal crosslinking with riboflavin and ultraviolet A for keratoconus. J Cataract and Refract Surg. 2007;33(11):1982-4. 9.Kohlhaas M, Spoerl E, Schilde T et al. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32(2):279-283. 10.Macsai MS, Varley GA, Krachmer JH. Development of keratoconus after contact lens wear: Patient characteristic. Arch Ophthalmol. 1990;108(26):534-8. 11.Mazzotta C, Balestrazzi A, Traversi C, et al. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen; ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea. 2007;26:390-7. 12.Meek KM, Tuft SJ, Huang Y et al. Changes in collagen orientation and dis-tribution in keratoconus corneas. Invest. Ophthalmol. Vis. Sci. 2005;46(6):1948-56. 13.Nowak DM, Gajecka M. The genetics of keratoconus. Мiddle East Afr. J. Ophthalmol. 2001;18(1):2-6. 14.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. 15.Rebecca McQuaid, Cummings AB, Mrochen M. The theory and art of corneal cross-linking. Indian J Ophthalmol. 2013;Aug; 61(8):416-9. 16.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp. Eye Res. 1998;66:97-103. 17.Spoerl E, Seiler TJ. Techniques for stiffening the cornea. Refract. Surg. 1999;15:711-3. 18.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385 9. 19.Sporl E, Huhle M, Kasper M, Seiler T. Increased rigidity of the cornea caused by intrastromal crosslinking. Ophthalmologe. 1997;94(12):902-6. 20.Tsubota K, Mashima Y, Murata H, Sato N, Ogata T. Corneal epithelium in keratoconus. Cornea. 1995;14:77–83. 21.Wollensak G, Spoerl E, Seiler T. Riboflavin ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620 7. 22.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29(9):1780–5.
|