J.ophthalmol.(Ukraine).2018;3:80-84.
|
https://doi.org/10.31288/oftalmolzh201838084 Structure of the chorioretinal complex in the rabbit eye after vitrectomy. Report 1. Vitreous cavity irrigation with different temperature solutions for 30 minutes O.S. Zadorozhnyy, Cand Sc (Med), R.E. Nazaretian, V.V. Myrnenko, V.A. Naumenko, Dr Sc (Med), Prof., E.V. Maltsev, Dr Sc (Med), Prof., N.V. Pasyechnikova, Dr Sc (Med), Prof. Filatov Institute of Eye Diseases and Tissue Therapy; Odessa (Ukraine) E-mail: laserfilatova@gmail.com TO CITE THIS ARTICLE: Zadorozhnyy OS, Nazaretian RE, Myrnenko VV, Naumenko VA, Maltsev EV, Pasyechnikova NV. Structure of the chorioretinal complex in the rabbit eye after vitrectomy. Report 1. Vitreous cavity irrigation with different temperature solutions for 30 minutes. J.ophthalmol.(Ukraine).2018;3:80-84. https://doi.org/10.31288/oftalmolzh201838084
Background: It remains poorly understood what should be the temperature of the irrigating solution for intraocular surgery and how long it is reasonable to use irrigating solutions during vitrectomy. Purpose: To investigate the structure of the rabbit chorioretinal complex after vitrectomy with the use of irrigating solutions having different temperatures for 30 minutes. Materials and Methods: Twelve Chinchilla rabbits (24 eyes) were divided into two experimental groups and one control group. The two experimental groups, each of 5 rabbits (10 eyes), underwent a three-port pars plana vitrectomy with either 22°C or 5°C irrigating solution. Two intact rabbits (4 eyes) were used as controls for comparison. The duration of irrigation/aspiration was 30 minutes. The material for histology was obtained at days 1 and 7 after surgery, and the chorioretinal complex was histologically examined by light microscopy. Results: Groups of animals subjected to thirty-minute vitreous cavity cooling with the 22 °C or 5°С irrigating solution showed no structural changes in the retinal and choroidal components compared to intact animals. Conclusion: The 22 °C or 5°С irrigating solution produces no structural changes in the retina and choroid, and can be used in the vitreoretinal surgical procedure for continuous vitreous cavity irrigation lasting for up to 30 minutes.
Keywords: vitrectomy, intraocular temperature, rabbit eye, chorioretinal complex
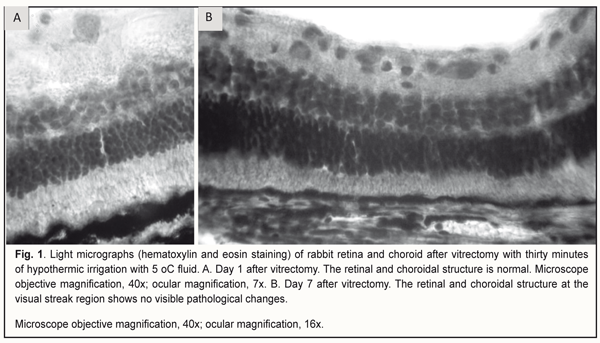
Introduction Therapeutic controlled hypothermia has been successfully applied in various medical fields (like cardiac surgery, neurosurgery, resuscitation science, and neonatology) for improving brain cell resistance to ischemic conditions [1-3]. The neuroprotective effect of hypothermia is based on reducing the induction of neuronal apoptosis through reduction in the rate of neuronal metabolism. Thus, a 1°С decrease in brain temperature leads to a 5% reduction in both neuronal oxygen consumption and glucose metabolism [4]. Irrigating solutions with a temperature well below that of intraocular media are used in common intraocular surgical procedures for a number of eye disorders. Usually, the temperature of the irrigating solution corresponds to that of the operating room environment and is not controlled during surgery [5]. Consequently, ophthalmological surgical procedures are performed under conditions of uncontrolled artificial local ocular hypothermia. Nevertheless, scant research has assessed the safety and efficacy of various temperature regimes in eye surgery [6-7]. It remains poorly understood what should be the temperature of the irrigating solution for intraocular surgery and how long it is reasonable to use irrigating solutions during intraocular surgery. Understanding the dynamics of structural changes in chorioretinal structures under conditions of hypothermia will enable (1) the development of a controlled ocular hypothermia technology, (2) more effective use of benefits of low temperatures in eye disease treatment, and (3) reductions in the rates of some intra- and post-operative complications. The purpose of the study was to investigate the structure of the rabbit chorioretinal complex after vitrectomy with the use of irrigating solutions having different temperatures for 30 minutes. Materials and Methods Twelve Chinchilla rabbits (24 eyes; weight, 2.5-3.5 kg) were included in this study and divided into two experimental groups and one control group. The two experimental groups, each of 5 rabbits (10 eyes), were subjected to vitrectomy with either 22°C or 5°C irrigating solution. The ambient operating room temperature was between 22 °С and 24 °С. Two intact rabbits (4 eyes) were used as controls for comparison. A 23-G three-port pars plana vitrectomy (PPV) was performed using the Alcon Accurus 400VS vitrectomy system (Alcon Laboratories, Fort Worth, TX). Technique The surgical site was prepared with antiseptic solution and epibulbar anesthetic was administered. Thereafter, a standard three-port core and peripheral vitrectomy was performed with cutting rates of 1500-1800 cuts/min, aspiration pressure of 150 mm Hg, and irrigation pressure of 20 mm Hg. The duration of irrigation/aspiration was 30 minutes. Balanced Ringer’s lactate was used as an intraocular irrigating solution. Cold (5 °С) fluid was prepared by cooling the solution inside the irrigating tube with gel packs that were located outside the tube, and thus cooling was performed in close proximity to the surgical site. Room-temperature (22 °C) fluid was obtained by placing the bottles with solution in the operating room for several hours before surgery. The temperature of the irrigating solution delivered into the eye was monitored and controlled during surgery. A thermoelectric device [8, 9] developed by the Institute of Thermoelectricity of the NAS of Ukraine and MES of Ukraine, and the Filatov Institute was used for measuring temperatures of various ocular structures, irrigating solution, and operating room. In addition, a smartphone attached infrared thermography system, FLIR ONE, (FLIR Systems, Wilsonville, OR) was used to monitor and control the temperature of the irrigating solution. After a lid speculum was placed, epibulbar anesthetic was administered, the three ports were created, and a temperature measuring probe was introduced into the vitreous cavity through a standard 23-G pars plana port. Temperatures in various parts of the vitreous cavity were measured before surgery and at different surgery time points. In addition, rectal temperature measurements were taken, and air temperature and relative humidity in the operating room were registered. All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV dated 21.02.2006 and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), and approved by a local Bioethics Committee of the Filatov Institute. The animals were housed and bred conventionally. Each animal underwent biomicroscopy and ophthalmoscopy at baseline, immediately after surgery, and then daily until euthanasia. Prior to surgery, animals were anesthetized with thiopental sodium 10% (1.0 mL/kg, intramuscularly). Immediately thereafter, both eyes received a drop of proxymetacaine HCl (0.5%) for topical anesthesia. The pupils were dilated with atropine sulphate. After surgery, a drop of sulfacyl natrium 20% and a drop of Ofloxacin 0.3% were applied to each eye four times daily during the observation period of 1 to 7 days. Histology was performed at the Pathology and Electronic Microscopy Laboratory of the Filatov Institute. Three animals from each experimental group of five rabbits were euthanized on the first postoperative day, and the remaining two rabbits in each experimental group were euthanized on the seventh postoperative day. Immediately after euthanasia of each rabbit, eye globes were enucleated and fixed for 24-48 hours in 10% neutral formalin. Formalin fixation was followed by processing, embedding in paraffin, sectioning to 5 μm, mounting and hematoxylin and eosin staining in a routine manner. The chorioretinal complex was histologically examined by light microscopy. Statistical analysis The experimental temperature data was subjected to statistical analysis. Data is presented as mean ± standard deviation (SD). Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. The level of significance p ≤ 0.05 was assumed. Results At baseline, mean rectal temperature was 37.98 ± 0.7 °С in the 22°C group and 38.2 ± 0.7 °С in the 5°C group (p = 0.6). In the 22°C group and the 5°C group, mean midvitreous temperature was 37.0±0.9 °С and 36.9±1.2 °С, respectively (p = 0.9), at baseline, and decreased to 25.8±0.5 и 10.9±1.4 °С, respectively, (p < 0.0001) after irrigation of the vitreous cavity during surgery. Therefore, compared to baseline, midvitreous temperature decreased by 11.2 °С and 26 °С (p < 0.0001), respectively. Mean vitrectomy time was 4 minutes, and mean irrigation/aspiration time was 30 minutes. In both groups, midvitreous temperature was not significantly different from baseline at 10 minutes after completion of irrigation. No corneal changes were observed, and lens clarity was maintained in all eyes during surgery. At day 1, histological examination found no loss of the normal layered structure of the retina in the animals of the 22°C group. In addition, no changes were found in (1) the number of cell rows in the outer nuclear layer and inner nuclear layer (5-8 rows and 3-4 rows, respectively), (2) width of these layers, and (3) structure of plexiform layers (the width of the INP was greater than that of the ONP). Although no marked edema was noticed, sporadic edematous cavities were observed at some locations (e.g., in the ganglion cell layer) on the slides. At day 7, the histological picture of the retina (and in particular, the medullary ray region) remained normal, and no changes were found in the structure of the choroid in the animals of the 22°C group. In addition, no edema was noted. Therefore, no histological changes in retinal and choroidal structure were found after thirty-minute cooling of the eye with the 22 °C irrigation solution. With regard to the 30-minute irrigation of the vitreous cavity with the 5 °C solution, at day 1, the structure of all the layers of the retina (and the structure of the choroid and sclera) remained normal, which was characteristic for the retinal sites located both in close proximity to and at varying distances from the optic nerve head. No apparent vacuolization of retinal structures was noticed. Single foci of vacuolization in the ganglion cell layer were observed at some locations on the slides. No other pathological changes were observed (Fig. 1A). Therefore, at day 1, the ocular structures were similar to those of intact animals. At day 7, the histology of the chorioretinal structure of the rabbits of the 5 °C group showed the following. The structure remained normal even in the visual streak in which the ganglion cell density is normally higher than elsewhere in the retina. The retina is adjacent to the choroid, and it was well seen that both the choroid and the visual streak had normal structure (Fig. 1B). In addition, no edema was noted. The retinal pigment epithelium (RPE) cells were full of melanin granules; this was especially well seen in the surface cuts of the RPE. The medullary ray had a normal structure, although it was rather thick (specifically, thrice as thick as the entire retina), which is characteristic of normal rabbit’s eye. Therefore, with regard to thirty-minute cooling with the 5 °C irrigation solution, the structure of the retina and choroid was still generally normal at the 7-day time point.
Discussion Pars plana vitrectomy has been firmly established as the gold standard of vitreoretinal surgery for diverse ocular pathology [10]. Despite advances in vitreoretinal surgical technology, there remain a wide range of challenges that affect the outcomes of treatment. Phototoxic damage and even thermal damage to the retina during vitrectomy has been reported. This is not surprising, since during vitrectomy procedures, the media provide no protection of the retina from the light of the endoilluminator [11]. In addition, toxic effects of dyes used during vitrectomy on the retinal neuroepithelium [12] and mechanical damage to the inner retina from air infusion during vitrectomy have been described [13]. Moreover, ocular hypoperfusion and ischemic retinal and optic nerve damage due to insufficient blood pressure and/or elevated IOP during vitrectomy have been reported [14]. Vitrectomy with long-term irrigation of the vitreal cavity with a low-temperature solution has been reported to produce retinal damage in rabbits. Thus, clinical and morphological changes in rabbit eyes after vitreoretinal surgery with three hours of hypothermic perfusion with either 22 °C or 2 °C lactated Ringer's solution were demonstrated by Zilis and colleagues in 1990. The mean preretinal temperature in the rabbits of the 22 °C group was 6.5 °C [7]. No significant histological changes (except for artifactually detached retina) were seen in either group at day 1. At day 7, electron microscopy and light microscopy found no structural changes in the retina or choroid of the 22 °C group (9 eyes). In addition, in the 2 °C group, seven of the nine eyes had obvious outer retinal and pigment epithelial damage (loss and disorganization of photoreceptors with macrophage infiltration accompanied by serous fluid accumulation), and some eyes demonstrated reversible lens opacities [7]. Therefore, a prolonged (3-hour) exposure to a cold (2 °C) irrigating solution in the vitreous cavity during vitrectomy procedure can lead to structural damage to the chorioretinal structures. The temperature of the intraocular media has been reported to approach that of the body under normal living conditions [5], which was also confirmed in our study. Thus, in animals of our 22 °C group, the body temperature was 37.98°С at baseline, and the midvitreous temperature dropped from 37 °C at baseline to 25.8°С (by more than 11°С) by the end of vitrectomy. Therefore, the use of a room-temperature (22-23 °С) infusion solution in vitreoretinal surgery results in a drop of intraocular temperature to deep hypothermia levels (below 30 °С) [2], which also confirms the need for development of recommendations on rates of cooling and re-warming of irrigating solutions during vitrectomy procedure. In our current study, thirty-minute cooling of the vitreous cavity with the 5°С irrigating solution during vitrectomy procedure resulted in a drop of midvitreous temperature from 36.9 °С to 10.9 °С (by 26 °С), but did not produce any structural changes in retinal and choroidal components compared to intact animals. Consequently, during vitrectomy, the irrigation solution temperature can be safely lowered to 5 °С for continuous vitreous cavity irrigation lasting for up to 30 minutes. Such temperature parameters can be used in some cases in vitrectomy for enhancing the resistance of nerve cells to ischemic conditions or when the structures of the chorioretinal complex are exposed to some other disturbing factors. In the study by Tamai and colleagues [6], after the rabbits underwent closed vitrectomy, their vitreous cavities were continuously irrigated with 8°С, 22°С or 38°С solution for 30 minutes at a perfusion pressure of 140 mm Hg to induce ischemia in the rabbit eye. At day 7, retinal damage in the 38°C group revealed more severe histological impairment than in either the 8°C or 22°C group. In addition, the least structural changes in the chorioretinal complex compared to controls were observed in the 8°C group [6], which is close to our findings. Therefore, developing a technology for controlled hypothermia of the eye requires further studies aimed at examination of best approaches for cooling the eye and search for the safest and most effective temperature and time regimens for cooling the intraocular structures. Conclusions First, in vitreoretinal surgery, the use of the 22°С or 5°С irrigating solution results in a drop of midvitreous temperature to 25.8°С or 10.9 °C, respectively (i.e., with intraocular temperature dropping to deep hypothermia levels). Second, no structural changes in the rabbit chorioretinal complex were found within seven days after vitrectomy with thirty-minute cooling of the vitreous cavity with the 22 °C or 5°С irrigating solution. Finally, the 22 °C or 5°С irrigating solution can be used in the vitreoretinal surgical procedure for continuous vitreous cavity irrigation lasting for up to 30 minutes. References 1.Alzaga AG, Cerdan M, Varon J. Therapeutic hypothermia. Resuscitation. 2006 Sep;70(3):369-80 2.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the ICU: Practical considerations, side effects, and cooling methods. Crit Care Med. 2009; 37: 1101–20 3.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56 4.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012 Feb 22;13(4):267-78 5.Iguchi Y, Asami T, Ueno S, et al. Changes in vitreous temperature during intravitreal surgery. Invest Ophthalmol Vis Sci. 2014 Apr 11;55(4):2344-9 6.Tamai K, Toumoto E, Majima A. Local hypothermia protects the retina from ischaemic injury in vitrectomy. Br J Ophthalmol. 1997 Sep;81(9):789-94 7.Zilis JD, Chandler D, Machemer R. Clinical and Histologic Effects of Extreme Intraocular Hypothermia. Am J Ophthalmol. 1990 Apr 15;109(4):469-73 8.Anatychuk LI, Pasyechnikova NV, Zadorozhnyy OS, et al. [A thermoelectric device for measuring intraocular temperature]. Thermoelektrika. 2015;3:31-40. Ukrainian 9.Anatychuk LI, Pasyechnikova NV, Zadorozhnyy OS, et al. [Original device and approaches to the study of temperature distribution in various eye segments (experimental study)]. Oftalmol Zh. 2015;6:50-3. Russian. 10.Machemer R, Parel JM, Norton EW. Vitrectomy: a pars plana approach. Technical improvements and further results. Trans Am Acad Ophthalmol Otolaryngol. 1972 Mar-Apr;76(2):462-6 11.Postel EA, Pulido JS, Byrnes GA, et al. Long-term follow-up of iatrogenic phototoxicity. Arch Ophthalmol. 1998 Jun;116(6):753-7 12.Farah M, Maia M, Rodrigues EB. Dyes in Ocular Surgery: Principles for Use in Chromovitrectomy. Am J Ophthalmol. 2009 Sep;148(3):332-40 13.Hasumura T, Yonemura N, Hirata A, et al. Retinal Damage by Air Infusion during Vitrectomy in Rabbit Eyes. Invest Ophthalmol Vis Sci. 2000;41:4300–04 14.Rossi T, Querzoli G, Angelini G, et al. Ocular perfusion pressure during pars plana vitrectomy: a pilot study. Invest Ophthalmol Vis Sci. 2014 Dec 2;55(12):8497-505
|