J.ophthalmol.(Ukraine).2018;3:3-9.
|
https://doi.org/10.31288/oftalmolzh2018339 Regional and central hemodynamics in ischemic optic neuropathy N.I. Khramenko, Cand Sc (Med), N.V. Konovalova, Dr Sc (Med), O.V. Guzun, Cand Sc (Med) Filatov Institute of Eye Diseases and Tissue Therapy; Odessa (Ukraine) E-mail: khramenkon@gmail.com TO CITE THIS ARTICLE: Khramenko NI, Konovalova NV, Guzun OV. Regional and central hemodynamics in ischemic optic neuropathy. J.ophthalmol.(Ukraine).2018;3:3-9. https://doi.org/10.31288/oftalmolzh2018339
Background: As ocular vascular disorders are a major cause of visual loss and affect working-age individuals, they are of particular social importance. Purpose: To investigate visual functions and ocular hemodynamics in ischemic optic neuropathy (ION) in the presence of changes in systemic and regional hemodynamics. Materials and Methods: Forty persons with ION underwent examination and treatment. Of these, 9 and 31 subjects had unilateral and bilateral ION, respectively. Patients underwent assessment of visual acuity, ophthalmoscopy, biomicroscopy, perimetry, intraocular pressure (IOP) and systemic blood pressure measurements, examination of the electrical sensitivity of the optic nerve and critical frequency of phosphene disappearance, ophthalmic rheography (ORG) and rheoencephalography (REG). Results: The data obtained indicate that there are correlations between regional blood flow characteristics and central hemodynamics parameters. This demonstrates once again that ION results from numerous etiologies, including systemic hemocirculation disorders, which agrees with the literature. In patients with low ocular pulse blood filling (OPBF), the mean pulse blood filling value for the internal carotid and vertebral-basilar systems was 71.4% lower (p < 0.05) than that in patients with normal OPBF. In ION, ocular hemodynamics insufficiency developed in the presence of increased diastolic pressure and reduced functional adaptive capabilities of the body. Conclusion: First, in the affected eye of patients with unilateral ION, the OPBF (expressed by the RQ index) was 44.4% lower, the vascular tone in small vessels was 17% increased (p < 0.05), and the ocular blood flow velocity was 39% decreased (p < 0.05) compared to those in the fellow eye. In patients with bilateral chronic ION, the RQ, the vascular tone of vessels of any size and ocular blood flow velocities were not significantly different from those from the affected eye of patients with unilateral ION. Second, in ION, best-corrected visual acuity (BCVA) was positively correlated with RQ (r = 0.31). If the RQ was lower than in age-matched normals, the BCVA was decreased to 0.5±0.06. Third, in patients with low OPBF, the mean pulse blood filling value for all brain artery systems was 71.4% lower (p < 0.05) than in those with normal OPBF. Fourth, in patients with low OPBF (i.e., OPBF lower than in age-matched normal controls), the mean diastolic blood pressure was 84.2±1.3 mmHg, which was 7.4% higher than those with normal OPBF (p < 0.05). Finally, adaptation potential characterizes the functional state of central regulation of the circulation system, and its value ranged from 2.1 units to 3.2 units and from 3.2 units to 4.3 units in 58.1% and 38.7%, respectively, of patients with ION, which evidences that adaptation mechanisms were subjected to some stress or functional reserves of circulation regulation were substantially depleted, respectively. Keywords: ischemic optic neuropathy, rheo-ophthalmogram, rheoencephalogram
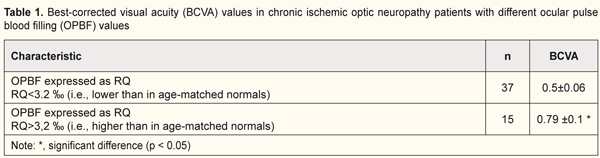
Introduction Ocular vascular disorders are a major cause of visual loss and affect working-age individuals. Ocular vascular occlusive disorders collectively constitute the most common cause of visual disability [1]. Ischemic optic neuropathy (ION) is a leading cause of blindness or severe visual impairment. Opinions vary with regard to the pathogenesis, clinical features and management of the disease, because ION is not one disease but a spectrum of several different types, each with its own etiology, pathogenesis, clinical features and management [2]. ION describes a state of hypoxic injury to any portion of the optic nerve from the optic chiasm to the optic nerve head [3]. Ischemia is a condition of an insufficient circulation in a portion of an organ, which is caused by a reduced arterial blood supply and results in inadequate blood supply to the tissue. A loss of cross-sectional area in medium-caliber vessels results in reduced blood flow velocity. Reduced intra-arterial pressure occurs due to reduced ocular arterial blood flow, redistribution of red blood cells, and reduced interstitial fluid reabsorbtion. Tissue metabolism becomes altered, which leads to hypoxia, dysregulation of oxidative phosphorylation, switching from aerobic to anaerobic respiration (glycolysis), accumulation of suboxidized metabolic products and lactic acid, pH shift to the acid side, and amino acid breakdown [4]. Clinically, ION is divided into anterior and posterior forms defined by the presence or absence of optic disc swelling, respectively [5]. Either form may occur in the presence or absence of vasculitis (arteritic or non-arteritic ION, respectively); practically all arteritic ION cases are giant cell arteritis [3]. It has been established that various forms of acute and chronic ION are not different nosologic entities but represent sequential stages in the same pathologic process [4]. Thromboembolism, systemic hypotension and atherosclerotic vascular occlusion are considered major factors [3] while hyperlipidemia, metabolic acidosis and blood hypercoagulation are confounding factors in the development of the disease [4]. The following factors have been highlighted as major contributors to the pathogenesis of ION based on electron microscopy evidence and investigation of blood flow in the optic nerve trunk: 1) structural features of the optic nerve head (ONH) and narrow scleral canal; 2) laminar and retrolaminar regions are the most common locations for infarction due to alterations in the blood supply in the short posterior ciliary arteries (SPCAs) [6]; 3) impaired blood supply in the ONH; 4) subadequate stability of the blood supply in the retrolaminar portion and choroidal blood supply in the posterior choroidal arteries; 5) presence of diabetes; 6) impaired autoregulation of the disc circulation by atherosclerosis, with a possible contribution from serotonin and endothelin-mediated vasospasm; 7) progression may be caused by secondary cell death after the initial ischemic insult or compression from cavernous degeneration and mechanical axonal distortion [7]. Chronic vascular insufficiency is characterized by markedly impaired circulation in anterior and posterior structures of the optic nerve [8]. It has been demonstrated that the main arterial vascular supply to the anterior optic nerve is from the SPCAs [9]. The morphologic appearance of the optic nerve after ischemic injury depends on several variables including the severity of insult and the time interval after the vascular event. The characteristic features of acute infarction are loss of cells and edema. Neutrophils are rarely present. Later, macrophages populate the peripheral area of infracted tissue. Severe ischemic injury results in profound cellular dropout, including loss of axons, myelin sheaths, and glial cells. Fibroblasts within pial septa are most resistant to ischemia, but their nuclei are usually small and pyknotic. Coagulative necrosis is the defining feature of all forms of ION [3]. Hamilton has identified the following phases in retinal vein occlusion [10]: 1. First 1-6 hours after occlusion (increased pressure in the proximal vein portion, endothelial dysfunction, increased vessel wall permeability, development of local retinal edema). 2. Six hours to one week after occlusion (the endothelium becomes disrupted, platelet adhesion develops at the newly exposed basement membrane, and the thrombus is formed, which results in microcirculatory stasis and hemorrhage). 3. One to five weeks (capillary occlusion persists, connective tissue proliferation emerges in the affected vessel, and the vessel becomes irreversibly occluded). Vascular insufficiency is bilateral in half of cases and is observed in individuals older than 60 years. The course of the disease lasts for more than 6 months. In the presence of slightly decreased visual acuity and concentric 5–10° narrowing of visual fields, ophthalmoscopy shows a swollen ONH with blurred margins and peripapillary atrophic rim (choroidal sclerosis), sclerotic artery walls and expanded veins. Subsequently, the ONH becomes pale, and its margins become distinct, which evidences the development of partial sclerotic atrophy of the optic nerve. Fluorescence angiography evidences choroidal perfusion delay in the peripapillary choroidal area. In most of these cases, rheography and Doppler confirmed calcification of the internal carotid siphon and carotid artery ischemia. In addition, in some patients, positron-emission computer tomography of the brain shows areas of cerebral hypoperfusion [8]. According to Eremenko [4], (a) sector papillitis, (b) vascular papillitis and retrobulbar neuritis, and (c) ischemic edema of the ONH represent sequential acute vascular optic neuropathy stages differing in severity, whereas chronic vascular insufficiency of the optic nerve, simple sclerotic atrophy of the optic nerve, and sclerotic atrophy of the optic nerve with pseudoglaucomatous cupping represent sequential chronic vascular optic neuropathy stages differing in severity. Hayreh distinguishes between two major types of ION, anterior and posterior ION (AION and PION, respectively). The former comprises arteritic AION (A-AION) and non-arteritic AION (NA-AION; this is due to causes other than giant cell arteritis). NA-AION is further classified into classical NA-AION and incipient NA-AION. PION consists of arteritic (A-PION - due to giant cell arteritis), non-arteritic (NA-PION - due to causes other than giant cell arteritis), and surgical (a complication of several systemic surgical procedures). Thus, according to Hayreh, ION actually consists of six distinct types of clinical entities [2]. Recently emerging information on the various factors that influence the optic nerve circulation, and also the various systemic and local risk factors which play important roles in the development of various types of ION have given us a better understanding of their pathogeneses, clinical features and management [2]. Currently, the thrombotic nature of vascular abnormalities is not questioned. In most cases, the underlying phenomenon is hypercoagulation. The role of these processes and whether thrombosis is a trigger or consequence of impaired blood circulation in the optic nerve vessels, however, remains unclear. Although the classifications based on these factors are heterogeneous, investigation of regional and systemic hemodynamics in ION is important. The purpose of this study was to investigate visual functions and ocular hemodynamics in ischemic optic neuropathy in the presence of changes in systemic and regional hemodynamics. Materials and Methods Forty persons (mean age, 56 ± 1.6 years; 17 men (55%) and 16 women (45%)) with ischemic optic neuropathy underwent examination and treatment at the Uveitis Department of the Filatov Institute. Of these, 9 and 31 subjects had unilateral and bilateral ION, respectively. Patients underwent assessment of visual acuity, ophthalmoscopy, biomicroscopy, perimetry, intraocular pressure (IOP) and systemic blood pressure measurements, and examination of the electrical sensitivity of the optic nerve and critical frequency of phosphene disappearance. In addition, they underwent ophthalmic rheography (ORG) and rheoencephalography (REG) with Reocom, the computerized rheography apparatus. ORG included measurements of ocular pulse blood filling (OPBF, expressed as RQ, ‰ rheographic coefficient) and vascular tone (expressed as alpha/T percentage index), whereas REG included assessment of relative pulse blood filling (expressed as relative rheographic index RI, i.e., ratio of the patient’s rheographic index to that of age-matched normals, taken as 100%) of brain vessels. The level of adaptation potential was calculated according to the formula developed by Baevsky [11]: AP = 0.011*HR + 0,014*SBP + 0.008*DBP + 0.09*M – 0.009*H + + 0.014*A – 0.27 where HR is heart rate per minute; SBP is systolic blood pressure, mmHg; DBP is diastolic blood pressure, mmHg; A is age, years; M is body mass, kg; and H is height, cm. The adaptation potential was considered satisfactory (with high or adequate functional capabilities) if the AP level was < 2.1. Adaptation mechanisms were considered to be subjected to some stress (with adequate functional capabilities ensured by functional reserves) if the AP level was within 2.11–3.2. The adaptation potential was considered unsatisfactory (with reduced functional capabilities) if the AP level was within 3.21–4.3. Finally, adaptation mechanisms were considered to be failed (with abrupt loss of functional capabilities) if the AP level was > 4.31. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. Data are presented as mean ± standard deviation (SD). A pairwise comparison was performed by using the paired Wilcoxon signed-rank test. Spearman's correlation coefficient was computed for all correlational analysis. Results and Discussion In vascular disorders of the optic nerve, dysregulation of circulation is observed at the local, regional and central levels [4]. In patients with chronic ION, best-corrected visual acuity (BCVA) was 0.58±0.05 (95% CI, 0.47 to 0.82) in the affected eye and 0.94±0.4 in the fellow eye. Mean IOP was 18.5±0.4 mmHg. BCVA was positively correlated with RQ (ocular pulse blood filling; r=0.31; p < 0.05). If the RQ exceeded that of age-matched normals (RQamn = 3.2‰), the BCVA was 0.79±0.1, and if the RQ was lower than RQamn, the BCVA was 0.5±0.06 (p < 0.05) (Table 1).
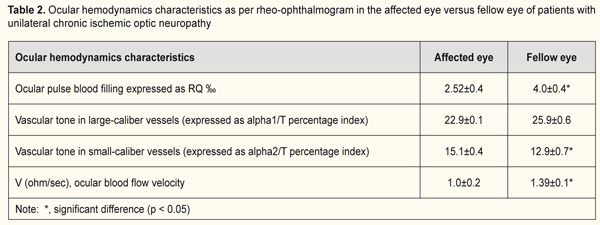
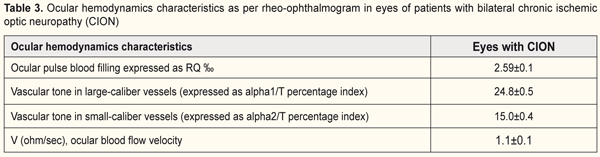
Ocular hemodynamics was assessed by ophthalmic rheography (ORG). In patients with unilateral ION, the RQ in the affected eye was 2.5±0.2‰ (i.e., 44.4% lower than that in the fellow eye (p < 0.05, Table 2). In addition, in the affected eye, the vascular tone in small vessels was 17% increased (p < 0.05), and the ocular blood flow velocity was 39% decreased (p < 0.05) compared to those in the fellow eye (Table 2). In patients with bilateral chronic ION, the RQ was 2.59±0.1 ‰ and was not significantly different from that from the affected eye of patients with unilateral ION (Tables 2 and 3). In addition, the vascular tone of vessels of any size and ocular blood flow velocities were not significantly different from those from the affected eye of patients with unilateral ION. In subjects with unilateral or bilateral ION, the RQ in the affected eye was 18.7% lower than that of age-matched normals (3.2±0.1‰; p < 0.05).
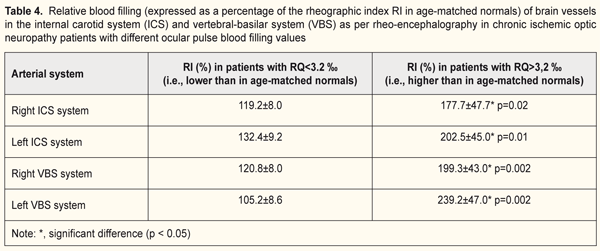
It has been reported that more than 20% of cases with vascular disorders of the optic nerve are associated with cerebral circulation disorders. Correlations have been found between optic nerve infarction and vascular injury to carotid or vertebral arteries [4]. In the current study, we examined relationships between local and regional circulation patterns, and found correlations between RQ and pulse blood filling of the internal carotid system (IBS) (r=0.41; p < 0.05), and between RQ and pulse blood filling of the vertebral-basilar system (VBS) (r=0.41; p < 0.05). In the majority of patients with ION (65.6%), the pulse blood filling in the vessels of the circle of Willis exceeded that of age-matched normals (Table 4; it this table, values are expressed as a percentage of age-matched normal controls). If an increase in pulse blood filling values for the vertebral-basilar and internal carotid systems was not hemodynamically significant (exceeded that of age-matched normals by 19.5%), the ocular pulse blood filling in the affected eye was insufficient (RQ <3.2 ‰). However, with the pulse blood filling values for the brain vessels exceeding those of age-matched normals by 104.7%, ocular hemodynamic values became normal (Table 4). On the whole, in patients with low OPBF, the mean pulse blood filling value for both brain artery systems was 71.4% lower (p < 0.05) than that in patients with normal OPBF (Table 4).
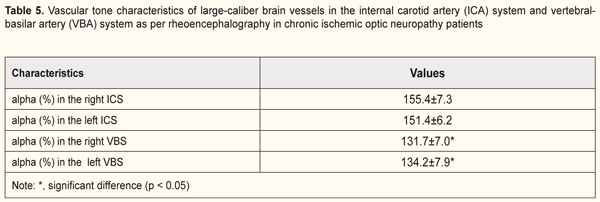
Therefore, in our opinion, increased pulse blood filling in the brain is a compensatory factor to allow for normalization of the ocular blood flow. In all patients with ION, in both cerebral hemispheres, the tone was 53.4% increased and 33% increased in large-caliber vessels of the ICS and VBS, respectively. Therefore, the tone in anterior cerebral compartments (in the ICS) was more increased compared to that in posterior cerebral compartments (in the VBS) (37% versus 13.7%, p < 0.05, Table 5).
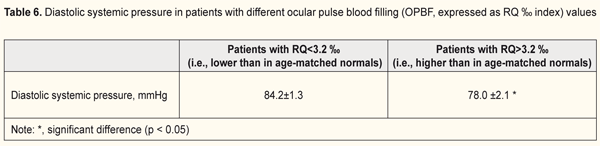
Given the literature data on central hemodynamics in patients with ION, we considered the influence of systemic blood pressure on ocular circulation (Table 6). Mean diastolic blood pressure (DBP) was 84.2±1.3 mmHg in eyes with insufficient ocular pulse blood filling (i.e., OPBF lower than in age-matched normal controls), which was 7.4% higher than in eyes with normal OPBF (p < 0.05, Table 6). Therefore, in ION, ocular hemodynamics insufficiency developed in the presence of increased DBP.
In the ischemic process, adequate functioning of the entire vascular system and adaptation of the system to new conditions are important and eventually reflect adaptation of the functional capabilities of the body. In our opinion, of the numerous characteristics discussed in the literature and proposed for health assessment, the index of functional changes in the circulatory system (or adaptation potential, AP) is the most suitable for use in practice. No loading tests are required to conduct before calculation of the AP that allows for a preliminary quantitative assessment of health for individuals [11]. For a long time, the treatment strategy for patients with chronic vascular impairments has been aimed only at improvement in hemodynamics (through reduction in cell oxygen demand and better control of oxygen delivery to cells). This treatment approach, however, does not protect cells from ischemic changes. Investigation of adaptive mechanisms of the body opens a new aspect in solving the problem. Adaptation mechanisms were found to be subjected to some stress or the adaptation potential of the central mechanisms of regulation of the vascular system was found unsatisfactory (with reduced functional capabilities) in most patients (58.1% and 38.7%, respectively) of this study (Table 7). Therefore, the above data demonstrates once again that that ION is a stage of generalized vascular pathology. In such patients, the risk of a vascular disaster (and that of ocular infarction) is substantially higher than in patients without alterations in brain circulation and/or systemic blood pressure, and, therefore, special attention should be given to diurnal variations in systolic and diastolic pressures, rheological state of blood, cerebral circulation, and adequacy of functional reserves of the body. In addition, more attention should be given to the medications that substantially improve metabolic abnormalities and are targeted at the mechanism of ischemic optic nerve disease in the presence of antisclerotic and vasodilating therapy.
Conclusions First, in the affected eye of patients with unilateral ION, the ocular pulse blood filling (expressed by the RQ index) was 44.4% lower, the vascular tone in small vessels was 17% increased (p < 0.05), and the ocular blood flow velocity was 39% decreased (p < 0.05) compared to those in the fellow eye. In patients with bilateral chronic ION, the RQ, the vascular tone of vessels of any size and ocular blood flow velocities were not significantly different from those from the affected eye of patients with unilateral ION. Second, in ION, BCVA was positively correlated with RQ (r = 0.31). If the RQ was lower than in age-matched normals, the BCVA was decreased to 0.5±0.06. Third, in patients with low OPBF, the mean pulse blood filling value for the ICS and VBS was 71.4% lower (p < 0.05) than in those with normal OPBF. Fourth, in patients with low OPBF (i.e., OPBF lower than in age-matched normal controls), the mean diastolic blood pressure was 84.2±1.3 mmHg, which was 7.4% higher than those with normal OPBF (p < 0.05). Finally, adaptation potential characterizes the functional state of central regulation of the circulation system, and its value ranged from 2.1 units to 3.2 units and from 3.2 units to 4.3 units in 58.1% and 38.7%, respectively, of patients with ION, which evidences that adaptation mechanisms were subjected to some stress or functional reserves of circulation regulation were substantially depleted, respectively. References 1.Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res. 2014 Jul;41:1-25 2.Hayreh SS. Ischemic optic neuropathy. Prog Retin Eye Res. 2009 Jan;28(1):34-62. 3.Patel HR, Margo CE. Pathology of Ischemic Optic Neuropathy. Arch Pathol Lab Med. 2017 Jan;141(1):162-6. 4.Eremenko AI. [Main forms of vascular optic neuropathies. (clinical picture, disgnostics and treatment)] [Abstract of. Dr Sc (Med) Dissertation]. Odessa: Filatov Institute of Eye Disease and Tissue Therapy; 1991. 29 p. Russian 5.Biousse V, Newman NJ. Ischemic optic neuropathies. N Engl J Med. 2015; 372(25):2428–36 6.Kerr NM, Chew SS, Danesh-Meyer HV. Non-arteritic anterior ischaemic optic neuropathy: a review and update. J Clin Neurosci. 2009 Aug;16(8):994-1000 7.Arnold AC. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2003 Jun;23(2):157-63 8.Morozov VI, Yakovlev AA. [Pharmacotherapy of eye diseases]. Fifth edition, revised and updated. Moscow: Meditsina; 2004. pp.339-44. Russian 9.Onda E, Cioffi GA, Bacon DR, Van Buskirk EM. Microvasculature of the human optic nerve. Am J Ophthalmol. 1995 Jul;120(1):92-102. 10.Budzinskaya MV, Mazurina NK, Egorov AE, et al. [Retinal vein occlusion management algorithm. Part 1. Classification, diagnosis, and acute-stage treatment]. Vestn Oftalmol. 2015 Nov-Dec;131(6):51-6. Russian
11.Baevsky RM. [Prediction of states on the verge of norm and pathology]. Moscow: Meditsina; 1979. pp.248-77. Russian
|
|