J.ophthalmol.(Ukraine).2018;2:11-16.
|
https://doi.org/10.31288/oftalmolzh/2018/2/1116 Relationships of vascular endothelial growth factor (VEGFA) with stage of diabetic retinopathy and disease duration in patients with type 2 diabetes mellitus A. S. Hudz1, Dr Sc (Med), G. Ie. Zakharevych, Assistant, O. V. Pentrenko2, Dr Sc (Med), S. V. Ziablitsev3, Dr Sc (Med), Prof., V. S. Dzhordzhua4, Ophthalmologist 1 Ophthalmology Department, Danylo Halytsky Lviv National Medical University; Lviv (Ukraine) 2 Ophthalmology Department, Shupik National Medical Academy of Postgraduate Education; Kyiv (Ukraine) 3 Bohomolets National Medical University; Kyiv (Ukraine) 4 Hospital Garcia de Orta EPE; Almada (Portugal) E-mail: anhudz@gmail.com TO CITE THIS ARTICLE: Hudz AS, Zakharevych GIe, Pentrenko OV, Ziablitsev SV, Dzhordzhua VS. Relationships of vascular endothelial growth factor (VEGFA) with stage of diabetic retinopathy and disease duration in patients with type 2 diabetes mellitus. J.ophthalmol.(Ukraine).2018;2:11-6. https://doi.org/10.31288/oftalmolzh/2018/2/1116
Background: As there is currently an epidemic of type 2 diabetes mellitus (T2DM), increasing attention should be paid to diabetic retinopathy (DR), a major complication of the disease which can lead to disability and blindness. Increased levels of growth factors (mainly, vascular endothelial growth factor (VEGFA)), angiogenesis factors, and cytokines in the aqueous humor have been reported in DR. Purpose: To identify the relationship of VEGFA with the stage of DR and duration of disease in T2DM through (1) investigation of the effect of increased aqueous VEGFA level on DR complications and (2) estimation of the sensitivity of clinical and laboratory indicators to the amount of VEGFA in the aqueous humor. Materials and Methods: Totally, 302 persons (604 eyes) were included in the study. Patients with T2DM were divided into three groups according to the severity of DR: Group 1 (no retinopathy, 76 patients), Group 2 (non-proliferative DR, 64 patients), and Group 3 (proliferative DR, 64 patients). The control group included 98 individuals with no T2DM or DR. Aqueous humor VEGFA levels were measured using enzyme-linked immunosorbent assay kits from eBioscience. Statistical analyses were performed using SPSS 11.0, MedCalc v.15.11.0 and Medstat Software. Results: Regression analyses demonstrated that aqueous VEGFA level directly and significantly influences the stage of DR and diabetes duration at baseline (P = 2.27E-08 and P =6.15E-05, respectively). In addition, increased aqueous VEGFA levels significantly influence the probability of developing neovascularization in the ocular tissue, vitreous hemorrhage or PDR (P < 0.05). An increase in the probability of developing optic disc neovascularization, retinal neovascularization, vitreous hemorrhage, vitreoretinal neovascularization, or PDR occurs with an increase in aqueous VEGFA level above 992 pg/mL, 923 pg/mL, 1005 pg/mL, 1022 pg/mL; and 926 pg/mL, respectively. Conclusion: Elevated aqueous VEGFA levels are a potent pathogenic factor for progression of DR in patients with T2DM. Key-words: diabetic retinopathy, vascular endothelial growth factor (VEGFA)
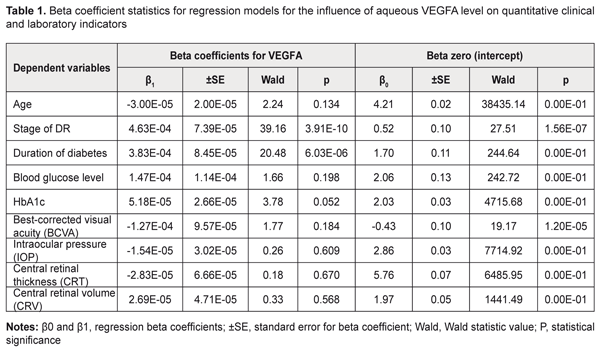
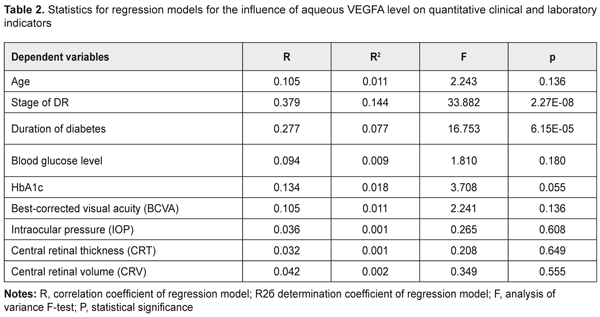
Introduction Like the rest of the world, in Ukraine, the prevalence of diabetic mellitus (DM) continues to increase, and for every previously identified patient with DM, three to four are newly diagnosed by screening every year [1-3]. The majority (80%) of patients with diabetic mellitus have type 2 diabetic mellitus (T2DM, i.e., noninsulin-dependent DM) [2, 4]. Major vascular diabetic complications include diabetic retinopathy and nephropathy as well as vascular injury to the lower extremity [1, 5]. Therefore, timely diagnosis, prevention and prediction of diabetic complications would contribute to halting further progression of the disease and to preventing ocular disability [1, 4]. As there is currently an epidemic of T2DM, increasing attention should be paid to diabetic retinopathy (DR) [4, 5]. In patients with type 2 diabetic mellitus, the prevalence of diabetic retinopathy increases with the duration of disease from 20% (newly diagnosed disease) to almost 50% (10 years of disease) [1, 2]. Globally, almost 93 million and 17 million patients with DM have been estimated to have non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR), respectively [5]. DR is a major diabetic complication that can lead to disability and blindness [4, 6]. Increased levels of growth factors and angiogenesis factors in the intraocular tissues have been reported in diabetic retinopathy [7, 8]. In addition, increased aqueous vascular endothelial growth factor (VEGFA) concentration plays a key role in the pathogenesis of diabetic vascular injury [9-11]. Although increased VEGFA expression has been identified as an important phase of the pathogenesis of DR, its mechanisms have been poorly investigated yet [9, 6, 12]. The purpose of the study was to identify relationships between VEGFA and the stage of DR and diabetes duration in T2DM through (1) investigation of the effect of increased aqueous VEGFA level on DR complications and (2) estimation of the sensitivity of clinical and laboratory indicators to the amount of VEGFA in the aqueous humor. Materials and Methods The study was conducted at the Ophthalmology Department of the Danylo Halytsky Lviv National Medical University. All procedures performed in the study were in accordance with the ethical standards of the Council of Europe's Convention on Human Rights and Biomedicine, the 1964 Declaration of Helsinki and its later amendments, and the Order of the Ministry of Health of Ukraine No. 690 dated 23 September 2009. Totally, 302 persons (604 eyes) were included in the study. Severity of DR was graded as per the 2003 guidelines of the American Academy of Ophthalmology. In each patient, left and right eyes showed no difference in the severity of DR. Patients with T2DM were divided into three groups according to the severity of DR: Group 1 (no retinopathy, 76 patients), Group 2 (NPDR, 64 patients), and Group 3 (PDR, 64 patients). No patient with T2DM had history of retinal laser photocoagulation or anti-VEGF therapy. The control group included 98 individuals with no T2DM, DR or any other ocular disease. Both cases and controls had a history of cataract surgery. The ophthalmological examination included visual acuity assessment; Goldman tonometry; slit lamp biomicroscopy (BQ 900; Haag-Streit, Köniz, Switzerland) of the anterior eye; gonioscopy and ophthalmoscopy with contact and noncontact lenses (Volk Optical, Mentor, OH); optical coherence tomography (OCT; Optovue, Inc., Fremont, CA); and fundus photography (the ETDRS seven standard fields) with the fundus camera TRC-NW7SF (Topcon, Tokyo, Japan). The best-corrected visual acuity (BCVA) was assessed and intraocular pressure (IOP, mmHg) was measured. In addition, central retinal thickness (CRT, μm) and central retinal volume (CRV, mm3) values were measured by OCT. Approximately 0.05 to 0.1 mL of aqueous fluid was aspirated by anterior chamber paracentesis using a 1.0-mL disposable syringe before cataract surgery. Aqueous humor VEGFA levels were measured using enzyme-linked immunosorbent assay (ELISA) kits from eBioscience (San Diego, CA). Aqueous humor samples were diluted at 1:2 for the kit using the assay diluents provided. Statistical analyses were performed using SPSS 11.0, MedCalc v.15.11.0 (MedCalc Software bvba, 1993–2015) and Medstat Software (Iu. Ie. Liakh, V. G. Gur’ianov, 2004-2012). Regression analysis only was needed for estimation of the effect of aqueous VEGFA level on quantitative variables. However, the effect of aqueous VEGFA level on qualitative variables should be (and was) estimated through the following steps. First, regression analysis was directed at identifying those dependent variables that were significantly influenced by the aqueous VEGFA level. Second, the optimal cut-off point was determined at which sensitivity and specificity were equal, and the significance and aqueous VEGFA level for this point were calculated. Univariate linear regression analysis was used to test for associations between aqueous VEGFA level and the quantitative variables. The data of T2DM patients with different DR stages were subjected to analysis. The aqueous VEGFA level was used as a predictor in the regression models. Dependent variables included the findings of the initial examination at presentation (age, stage of DR, diabetes duration, blood glucose level, HbA1c, BCVA, IOP, CRT and CRV). Generalized Linear/Nonlinear Models (GLZ) procedure in the SPSS was used to develop a regression model for each not normally distributed dependent variable. The comprehensive statistical assessment of the significance of the influence of the aqueous VEGFA level on the dependent variables included analysis of the significance of beta coefficients of regression equations and analysis of the significance of regression models in general. Univariate logistic regression analysis was used to test for associations between aqueous VEGFA level and the qualitative clinical and laboratory indicators. The data of T2DM patients with different DR stages (Groups 1 to 3) were subjected to analysis. Dichotomous categorical variables (sex, compensated diabetes, macular edema, optic disc neovascularization, retinal neovascularization, vitreous hemorrhage, vitreoretinal neovascularization, and PDR) were used as dependent variables coded as 1 = Yes and 0 = No. The aqueous VEGFA level was used as a predictor in the analyses. When building logistic models, we used maximum likelihood estimation method with the comprehensive statistical assessment of the validity of regression models which included analysis of the significance of beta coefficients of regression equations and analysis of the significance of regression models in general. The level of significance P ≤ 0.05 was assumed. Results and Discussion Tables 1 and 2 present the results of the analyses for the quantitative clinical and laboratory indicators. The following model fitness criteria were used: the beta coefficients have statistical significance (by the Wald test) compared with the null hypothesis; the F test is significant; and the coefficient of determination (R2) is significant.
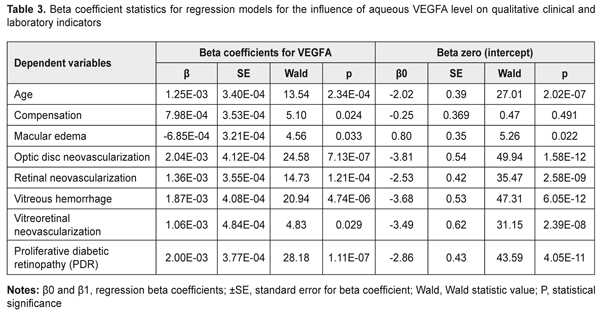
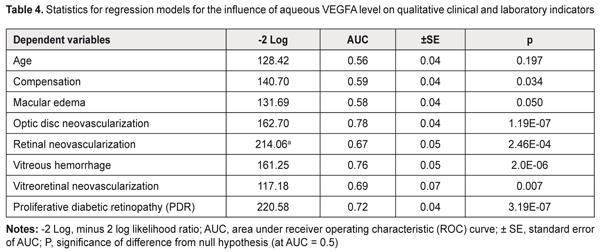
The dependent variables in the regression equations, the stage of DR and diabetes duration, showed satisfactory performance. Given the mathematical expression of the models, one may conclude that aqueous VEGFA level directly and significantly influences the baseline stage of DR and baseline diabetes duration (P = 2.27E-08 and P =6.15E-05, respectively): Y=β0+β1*VEGFA(1), where Y is the dependent variable, β0 is an intercept, β1 are beta coefficients for VEGFA; VEGFA is aqueous VEGFA level. Tables 3 and 4 present the results of the analyses for the qualitative clinical and laboratory indicators. The following model fitness criteria were used: the intercept as well as beta coefficient for the predictor variable has statistical significance (by the Wald test) compared with the null hypothesis; the ROC curve AUC is significant compared with the null hypothesis; and the AUC value (the test is considered a failure if AUC < 0.6).
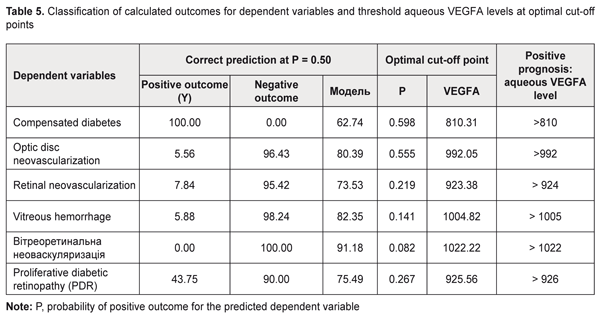
Based on the criteria above, we selected for further analysis the models that define the probability (P) of occurrence of the following events (categories) in the future: optic disc neovascularization, retinal neovascularization, vitreous hemorrhage, vitreoretinal neovascularization, and PDR (P < 0.05). The following is the general formula for the models: P=1/(1+e-(β0+β1*VEGFA))(2), where: P is a probability of a positive outcome for the categorical variable, β0 are intercepts, β1 are beta coefficients for VEGFA; VEGFA is aqueous VEGFA level. It should be noted that choosing a cut-off point on the probability curve, i.e., determining a probability threshold at which one of the binary outcomes is assigned, is very important in two-class classification problems (e.g., the problem considered here). Making a choice of the cut-off point makes it possible to control the probability of correct identification of positive and negative outcomes. In addition, in a binary model, there is an optimal cut-off point at which sensitivity and specificity are equal, and correctness of the prediction is the same for positive and negative outcomes. The analysis of the logistic regression models of relationships between qualitative clinical and laboratory indicators and aqueous VEGFA level demonstrated the following optimal cut-off points for the development of optic disc neovascularization, retinal neovascularization, vitreous hemorrhage, vitreoretinal neovascularization, or PDR: P = 0.555, ; P = 0.219; P = 0.141; P = 0.082 and P = 0.267, respectively. The formula for calculating the probability of the development of positive outcome for a categorical variable (2) is given above. The second purpose of our study was solving an inverse problem, i.e., identifying a threshold aqueous VEGFA level at which a variable category would take values corresponding to the positive outcome. In this connection, we obtained the following formula (that is inverse to formula (2)) for calculating the threshold aqueous VEGFA level based on the event probability: VEGFA=(-ln(1/P-1)-B0)/B1(3), where: P is a probability of a positive outcome for the categorical variable, β0 are intercepts, β1 are beta coefficients for VEGFA; VEGFA is aqueous VEGFA level. The probability of correct identification of the occurrence of the resulting event increases with increase in the threshold value, whereas the probability of correct identification of the non-occurrence increases with decrease in the threshold value. Table 5 presents classification of the results of regression analysis. We found that an increase in the probability of developing optic disc neovascularization, retinal neovascularization, vitreous hemorrhage, vitreoretinal neovascularization, or PDR occurs with an increase in aqueous VEGFA level above 992 pg/mL, 923 pg/mL, 1005 pg/mL, 1022 pg/mL; and 926 pg/mL, respectively.
Conclusion First, our regression analyses demonstrated that aqueous VEGFA level directly and significantly influences the stage of DR and diabetes duration at baseline (P = 2.27E-08 and P =6.15E-05, respectively). In addition, increased aqueous VEGFA level significantly influences the probability of developing neovascularization in the ocular tissue, vitreous hemorrhage or PDR (P < 0.05). Second, an increase in the probability of developing optic disc neovascularization, retinal neovascularization, vitreous hemorrhage, vitreoretinal neovascularization, or PDR occurs with an increase in aqueous VEGFA level above 992 pg/mL, 923 pg/mL, 1005 pg/mL, 1022 pg/mL; and 926 pg/mL, respectively. References
|