J.ophthalmol.(Ukraine).2018;2:60-66.
|
https://doi.org/10.31288/oftalmolzh/2018/2/6066 Removal of Persisting Pupillary Membrane with Refractive Lens Exchange N. F. Bobrova, Dr. Sc. (Med.), Prof.; A. V. Artemov, Cand. Sc. (Med.); T. V. Romanova, Cand. Sc. (Med.); D. V. Smaglii, an internship doctor SI “Filatov Institute of Eye Diseases and Tissue Therapy of NAMS of Ukraine”; Odessa (Ukraine) E-mail: filatovbobrova@gmail.com TO CITE THIS ARTICLE: Bobrova NF, Artemov AV, Romanova TV, Smaglii DV. Removal of Persisting Pupillary Membrane with Refractive Lens Exchange. J.ophthalmol.(Ukraine).2018;2:60-6. https://doi.org/10.31288/oftalmolzh/2018/2/6066
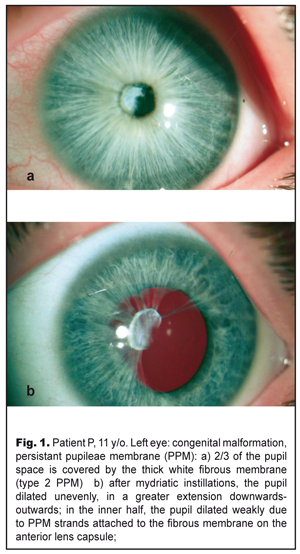
Background. Clinical characteristics, histological structure, and surgical technique of persisting pupillary membrane (PPM) have been presented in the modern literature in insufficient and, in some cases, contradictory reports. Purpose. To describe clinical characteristics and histological structure of PPM, as well as PPM surgery for restoring the transparency of the optic axis and eliminating anisometropia through refractive lens exchange. Material and Methods. Complete ophthalmic examination including ultrasound biometry and scanning followed by surgical removal of PPM and refractive exchange of the clear lens was performed in an 11-year-old patient with high myopia, anisometropia and myopia Results. The PPM case reported is a combination of multiple strands, aligning with the iris in color, involved in the stroma and the inner half of the iris and attached to the anterior lens. The strands, thus, obscured 2/3 of the iris. By sequential surgical dissection of the PPM strands, the membrane was detached from the anterior capsule and removed from the eye. Refractive lens exchange was performed using a standard technique. Histological studies showed a dual character of the removed PPM: the presence of both thick fibrous connective tissue and loose fibrous tissue, resembling the stroma of the iris with the presence of collapsed capillaries. Conclusions. 1. The rare clinical case described is an example of combined type 1 and type 2 PPM according to Duke-Elder’s classification (1964) or a combination of accessory iris membrane and persistant pupilеae membrane. With the point of Duke-Elder’s classification, this membrane can be considered a special variant of type 2 PPM whereas there is no connection with the lens capsule. 2. Attachement of PPM to the anterior lens capsule, which seemed quite tight, was false since PPM, after being dissected, spontaneously detached from the lens with the latter preserved intact and transparent, which contributed to recover the transparency of the optic axis. 3. One-stage refractive replacement of the lens made it possible to eliminate high myopia and high anisometropia, which made it possible to increase visual acuity of the eye and to create maximal favorable conditions for amblyopia treatment that followed. Key-words: persistant pupilеae membrane, refractive lens exchange, children Introduction Persistent pupillary membrane (PPM) develops as a result of embryogenesis disorders, which is a reverse development of tunica vasculosa lentis that supplies nutrition to the lens in the first six month of fetal life [9]. Involution of the central part of the membrane pupillaris occurs at seven or eight months of gestation. Persisting pupillary membrane is observed when this atrophy is incomplete [5]. The reserve development of the membrane also occurs after an infant is born, more intensively during the first year of the life [8]. Remnants of membrane pupillaris as PPM vary in a wide range beginning from thin cobweblike strands seen on a slit lamp to dense straining membranes covering a pupil space and inducing amblyopia [1]. Membrane strands can be connected with the cornea or the lens, but more often with other parts of the iris and between themselves [9]. Incidence of PPM is quite large ranging from 30 to 95% of healthy individuals [1, 8]. Herewith, in most cases, thin strands of the membrane do not affect visual acuity, do not require any treatment, and can be unidentified by the individual. Treatment is required when PPM obscurs the optic zone and visual acuity decreases, which in childhood can be a cause of amblyopia. [2, 14]. We report the case of an 11-year-old adolescent with PPM in an eye with high myopia and anisometropia having led to severe amblyopia. The purpose of the present paper was to describe clinical characteristics and histological structure of PPM, as well as PPM surgery for restoring the transparency of the optic axis and eliminating anisometropia through refractive lens exchange. Material and Methods An 11-year-old adolescent P. was presented at the Ukrainian Centre of Pediatric Ophthalmic Pathology at the Filatov Institute of Eye Diseases and Tissue Therapy. The patient was diagnosed with congenital malformation, PPM, high myopia, anisometropia, and high myopia in the left eye; and emmetropia in the right eye. BCVA OS=0.02–0.03; BCVA OD=1.0. According to the history, patient’s mother repeatedly referred to different ophthalmologists for poor vision in the left eye; however, the patient underwent no treatment. At presentation: the left eye was calm with a transparent and brilliant cornea. The anterior chamber was of an average depth, even and with transparent aqueous humor. A pattern of the iris was smoothed. The pupil was round, 2.5 in diameter, centrally located; 2/3 of the iris were occupied by PPM in a form of a dense fibrous white membrane, 2.5-3.5 mm in diameter, which was tightly attached to the anterior capsule of the lens, occupying 2/3 of the optic zone. An outer half-moon-shaped third of the iris was free (Fig. 1a). The pupil in the inner half dilated unevenly, more extensively downwards-outwards (Fig. 1b), since the PPM strands were strained and there was a fibrous film on the anterior lens capsule which impeded full dilation of the pupil. The lens in the ophthalmoscopically available zone was transparent. Eye fundus: the optic disc was palish, clearly outlined, and surrounded by a thin edge of pigmentation; vessels were narrow; macular reflex was poor; periphery was unchanged.
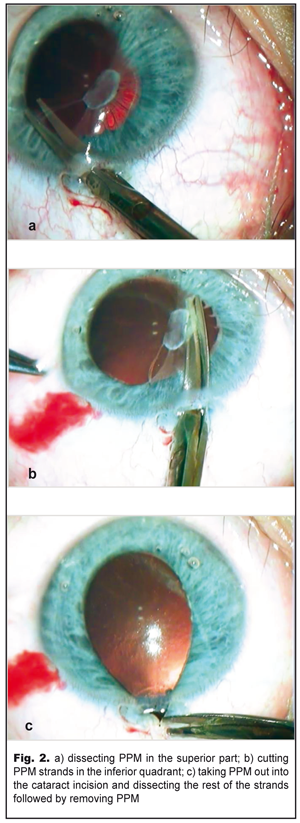
UCVA OS=0.02-0.03; UCVA OD=1.0. Gonioscopy showed in the left eye goniosynechiae on the inside of the anterior chamber angle. IOP OD=21 mmHg; IOP OS= 18.0 mmHg. Tonography: OD 5.5: Р = 13.6; С=0.25; F =0.84; Р0/С =53; OS 5.5: Р = 14.2; С=0.2; F =0.91; Р0/С =73 Ultrasound distance biometry: OD: 3.35 4.01 23.41 mm; OS: 3.12 4.26 26.15 mm. Ultrasound scanning OS: sonography revealed the anterior chamber was of an average deapth, 3.2 mm; the anterior chamber angle of 47˚; single punctate indurations in the lens, 3.6 mm thick, of decreased echogenicity. The papillary edge of the iris was connected with the anterior lens capsule by punctate fibrous structures. The ciliary body was attached. No pathological changes were revealed in the posterior eye. Perimetry: OD: visual field in norm; OS: visual field loss was 10° inside and 20° outside. Refractometry: OD: emmetropia; OS: myopia (-8.0D) Keratometry: OD: 42.0 D; OS: 41.75D Acrysof SN60WF IOL calculations: OD: 22.4D; OS: 15.76D Considering the presence of PPM and high myopia, the patient underwent the removal of PPM with the refractive replacement of the lens and intracapsule IOL implantation in the left eye in order to recover the transparency of the optic axis and to eliminate anisometropia. Excised PPM fragments were histomorphologically studied using hematoxylin and eosin staining. Operational technique: 1% phenylephrine was administered into the anterior chamber; the pupil, therefore, dilated unevenly due to PPM strands going from iris collarettes and attaching to the thick fibrous film on the lens. Anterior-chamber scissors inserted into a 2.2 mm cataract incision were used for visco-dissection of membrane strands from the lens and, afterwards, the membrane strands were dissected, at first, superiorly (Fig. 2a) (leaving some strands uncut) and, then, internally reaching 6 o’clock along the pupillary edge of the iris (Fig. 2b). Herewith, we noted small hemorrhages in the dissection sites of the thickest strands, which indicated the occurrence of persisting vessels in them.
After inferior strands were completely dissected, the thick part of the membrane, which seemed to be tightly attached to the anterior lens capsule, self-shifted upwards to the site of the strands left uncut. Thus, using forceps through traction, the membrane with the strands remained in the papillary edge of the iris was peeled outwards through the incision and dissected (Fig. 2c). Afterwards, the membrane with the strands was removed and the iris self-filled up the anterior chamber. The lens remained intact; the pupil, freed from synechiae, dilated spontaneously.
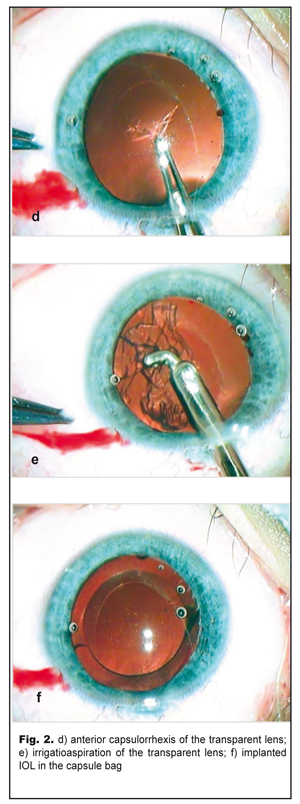
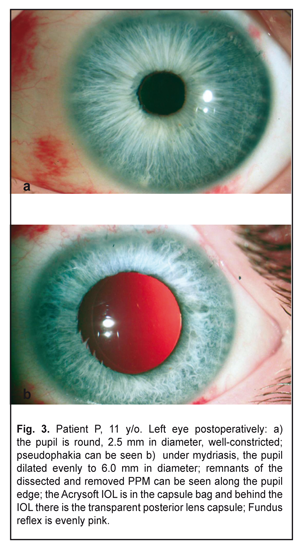
On completion of d=5.5 capsulorrhexis (Fig. 2d) and phacoaspiration of the clear lens (Fig. 2e), an IOL, SN60WF 16.0 D, was implanted into the capsule bag using a cartridge (Fig. 2f). The pupil narrowed symmetrically in response to myotic administration into the anterior chamber; viscoelastic was aspirated and the incision was sutured. The postoperative period was without abnormalities. Results and Discussion The left eye was calm with a clear brilliant cornea, an average depth anterior chamber, and transparent aqueous humor. The round pupil, in the center, constricted well (Fig. 3a) and evenly dilated by mydriatics to 6.0 mm; herewith, bases of the dissected PPM strands could be seen along the inner edge of the pupil (Fig. 3b). The Acrysof IOL was correctly placed in the capsule bag; behind the IOL there was a transparent anterior capsule. The eye fundus reflex was even and pink with details in norm. IOP was normal.
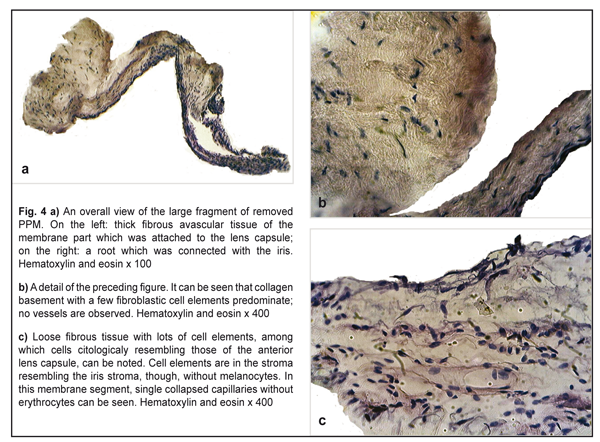
Postoperatively, BCVA increased to 0.1 by the Shevalev’s chart and to 0.25 by Teller cards. Gavris et. al. believes that accessory iris membrane (AIM) and persistant pupilеae membrane (PPM) should be differentiated. Both conditions are congenital iris anomalies which in a form of fine or thick iris strands arise above the iris surface and obscure the pupil. In addition, AIM, which is sometimes called iris duplication, very resembles the iris itself in color and structure and has a virtual second pseudopupil in the center while PPM is a translucent or opaque membranous structure which is extended across the pupil and has no pseudopupil [10]. In 1998, Bhatti reported two cases of binocular AIM in the eyes with microcornea [7] while, in 1979, Suh described two cases of AIM in the eyes without concomitant congenital anomalies [21]. From this point of view, our clinical case has a combination of AIM (fine strands of the iris color with vessels) and PPM (thick white membrane on the anterior lens surface that is attached by the iris strands). A similar combination was reported by Levy in 1957 [17]. It should be noted that Duke–Elder’s classification of persistant pupilеae membranes differentiate three types of PPM: PPM which starts at the iris and is attached to the iris (type 1), the lens (type 2), and the cornea (type 3) [9]. So, it is obvious that marking any other subtypes is quite unnatural since all the membranes mentioned are of the same origin and morphological structure which is mesodermal tissue [3]. Thus, accessory iris membrane can be referred, according to Duke–Elder’s classification, to type 1 PPM while persistant pupilеae membrane is referred to type 2 PPM. So, our case is a combination of type 1 and type 2 PPM, which is logical considering embryogenesis stages and histological structures of fragments of the removed membrane. Thus, histomorphologic studies of the dissected membrane fragments showed that it contained two components. One of them was mainly thick fibrous connective tissue (Fig. 4a and 4b); another one was loose fibrous tissue resembling the corneal stroma (Fig. 4c). Both components had no signs of active vascularization; collapsed capillaries, though, could be seen among elements in the loose fibrous tissue but without erythrocytes (Fig. 4c). Particular attention should be paid to the membrane component which was attached to the anterior lens capsule but not tightly as appeared during the surgery. Figures 4a and 4b demonstrate that this component is similar in structure to that thick fibrous tissue which can be formed by corneal epithelium and endothelium, and lens epithelium in the presence of pathological over-growth in the eye [6]. The absence of connection with the lens capsule indirectly points at the fact that this fragment of the membrane was formed after lens placode had been separated and the lens vesicle had been formed. In addition, this membrane fragment was formed from that part of “neuroectodermal mesenchyme”, the cells of which participated in the corneal stroma formation. That is why, if this membrane has anatomic and topographic signs of type 2 PPM according to Duke-Elder classification, then histological structure of tissue anterior to the lens points at its histologic similarity to the corneal stroma. From this point of view, the given case can be considered as pseudo-PPM of type 3, i.e. close to type 3 PPM in histological structure and to type 2 PPM anatomically and topographically. PPM is recommended to treat only when it affects visual acuity; in this case visual acuity should be tested in the bright lighting condition.
The treatment recommended is both surgical (PPM dissection and removal, as reported in our case) and laser [4, 10, 15, 18, 19, 20]. Moreover, according to the literature data [10, 13, 16, 22], laser treatment is more preferable for AIM and type 1 PPM when a membrane has a pseudo-pupil and is attached to the iris only; surgical treatment is recommended for the presence of PPM or lens membranes of type 2 PPM. Herewith, after membrane removal, it is often that a surgeon must perform cataract extraction since a damage of the lens capsule is the most common complication [11, 12]. Our previous papers [2-4] have reported that delicate manipulations using enough viscoelastic and microinstruments enable to peel PPM from the anterior capsule with the latter preserved intact. Consequently, the lens is preserved transparent, which is crucial, especially in childhood, and makes it possible to recover both vision and accommodative capability of the native lens, considering the following refractogenesis of a developing eye. In the case reported, the integrity of the anterior capsule and, consequently, the transparency of the lens were also preserved; and the lens was removed with a refraction purpose to eliminate high anisometropia associated with high myopia. Conclusion 1.The rare clinical case described is an example of combined type 1 and type 2 PPM according to Duke-Elder’s classification (1964) or a combination of accessory iris membrane and persistant pupilеae membrane. With the point of Duke-Elder’s classification, this membrane can be considered a special variant of type 2 PPM whereas there is no connection with the lens capsule. 2.Attachement of PPM to the anterior lens capsule, which seemed quite tight, was false since PPM, after being dissected, spontaneously detached from the lens with the latter preserved intact and transparent, which contributed to recover the transparency of the optic axis. 3.One-stage refractive replacement of the lens enabled to eliminate high myopia and high anisometropia, which made it possible to increase visual acuity of the eye and to create maximal favorable conditions for amblyopia treatment that followed. References
|