J.ophthalmol.(Ukraine).2018;2:50-55.
|
https://doi.org/10.31288/oftalmolzh/2018/2/5055 Modelling form deprivation myopia in experiment I. N. Mikheytseva, Dr Sc (Biol); Mohammad Abdulhadi; A. A. Putienko, Dr. Sc. (Med.); A. G. Kovalchuk, Cand. Sc. (Med.); S. G. Kolomiichuk, Res Fellow; T. I. Siroshtanenko, Jr Res Fellow Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine; Odessa (Ukraine) E-mail: filatovbiochem@ukr.net TO CITE THIS ARTICLE: Mikheytseva IN, Mohammad Abdulhadi, Putienko AA, Kovalchuk AG, Kolomiichuk SG, Siroshtanenko TI. Modelling form deprivation myopia in experiment. J.ophthalmol.(Ukraine).2018;2:50-5. https://doi.org/10.31288/oftalmolzh/2018/2/5055
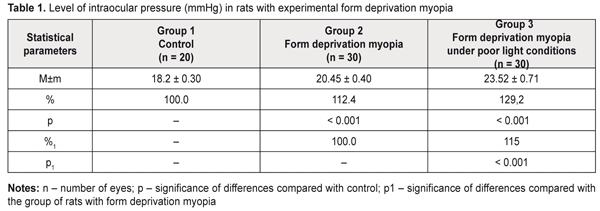
Background. Myopia, leading to visual function impairment, is one of the most topical issues in eye pathology. Although myopia is a common disease, its pathogenesis has a set of questions unstudied, which is why it is crucial to develop an axial myopia model on animals, a clinical picture of which is maximally similar to human and makes it possible to study both pathogenesis and treatment of myopia. So, the purpose of the present paper was to study certain parameters of the visual organ when modelling form deprivation myopia in rats under different light conditions. Material and Methods. Group 1 (10 animals) consisted of intact rats. Group 2 (15 animals) consisted of the rats, both eyelids of which were sutured to induce form deprivation myopia. Group 3 (15 animals) were rats with eyelids sutured to induce form deprivation myopia which, unlike Group 2 rats, were kept under poor light conditions for 14 days. Within the same period, animals of Groups 1 and 2 were kept under natural light conditions. Once a 14-day period expired, sutures were taken out from the eyelids. After suture removal, all the animals were performed ultrasound scanning, tonometry (Maklakov applanation tonometer, a 2 g and 4 mm plunger) to measure intraocular pressure, and pachymetry (Handy Pachymetr SP–100) to measure corneal thickness. At two weeks after removal of sutures from the eyelids, the animals were sacrificed under general anesthesia and the eyeballs were enucleated. Objective criterion for myopia development was elongation of anterior-posterior dimension (APD) of the eyeball which was measured using ultrasound scanning (in vivo) and a digital sliding caliper (Topex) with 0.02 mm accuracy (post mortem). Data obtained were processed using the non-parametric Kruskall-Wallis and Mann-Whitney tests using a software program (Statistica 5.5). Results. Our data on changes in visual organ parameters (intraocular pressure, corneal thickness, anterior-posterior dimension of the eyeball) in young rats when modelling form deprivation myopia under different light conditions showed more rapid progression of myopia when lighting was poor. Thus, the changes in the parameters involved in myopization were significantly more pronounced in the rats with an axial myopia model induced under poor light conditions as compared to those in experimental myopia under natural lighting (IOP was higher by 15.0%; APD was longer by 5.0%). Conclusions. Form deprivation myopia which is induced by eyelid suture in two-week-old rats in the period of eyeball growth under poor light conditions can be recommended for studying structural and functional characteristics of progressive myopia and developing pathogenetically-oriented treatment methods for the disease. Key-words: form deprivation myopia, lighting, ultrasound scanning, tonometry, pachymetry, rats, experiment Background Myopia, leading to visual function impairment, is one of the most topical issues in eye pathology. Social significance of myopia is important since the disease can develop in school and teenage children and rapidity of disease progression leads to limited choice of professional specialization and incapacitation [1–3]. Although myopia is a common disease, its pathogenesis has a set of questions unstudied, which is why it is crucial to develop an axial myopia model in animals whose clinical picture is maximally similar to human. Investigations in such a model will enable to study both pathogenesis and treatments of myopia. Currently, myopia models of different complexity which can be induced in young animals have been developed [4–6]. There is a model of myopia progression with fibroblast transplantation on sclera [7]. In a number of studies, a relationship of accommodation disorders and hemodynamic processes with structural and optical changes in the eyes with progressive myopia has been shown [8–10]. Progressive myopia can be modelled by reducing a level of regional hemodynamics and short-term increasing the intraocular pressure through administration of nicotinic acid into rabbits during their growth period [11, 12]. Another model of myopia in rabbits is by papain solution administration under the conjunctiva into equator area in four segments [13]. Of a special interest is a pathogenetically substantiated form deprivation model of extreme myopia in chicks using spectacles (-20D), an essential fault of which is a bilaminar scleral structure of a chick eye [14]. The most sensitive period for development of experimental form deprivation myopia in chicks is first days of life. In rats, this period starts at 15th Day when their eyelids are open [15]. It is known that retinoic acid ingestion in chicks also induces elongation of the eyeball [16]. In a form deprivation myopia model in guinea pigs and primates, retinoic acid levels in the retina and choroid correlated with ocular elongation [17]. Experimental studies have proved that the peripheral retina plays an important role in the development of form deprivation myopia. Moreover, defocus of the eye makes it myopic; defocus develops independently of accommodation and under local control and is followed by local changes in the choroid and sclera [18, 19]. Nowadays, retinal neurotransmitter receptors are believed to stimulate the development of experimental form deprivation myopia. Some authors have reported on the retina (Muller cells, to be more precise) as an eyeball growth inductor [20]. However, most of the models described are difficult to access. That is why, as a basis, we used a model of form deprivation myopia induced by tarsorrhaphy of the right eye in the period of intensive eyeball growth [5]. This model has been described in regard to a character of structural and functional disorders in eyeball tissues [21] and structures of scleral collagenic fibrils [22, 23] – scleral thinning and degenerative changes in the retina [19, 23, 24]. In addition, we experimentally studied an effect of overburden modelling conditions, keeping animal in poor light conditions, in particular, since there is evidence showing that eyeball growing under poor lighting can contribute to axial elongation [25–27]. The purpose of the present paper was to study certain parameters of the visual organ when modelling form deprivation myopia in rats under different light conditions. Material and methods The experiment involved two-week-old Wister rats and followed the General Ethical Principles of Animal Experiments (approved by the Third National Congress on Bioethics Ukraine, Kyiv, 2007) and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986). Group 1 (10 animals) consisted of intact rats. Group 2 (15 animals) consisted of the rats in which both eyelids were sutured to induce form deprivation myopia (Beuerman R.W. et al., 2010) [5]. Group 3 (15 animals) were the rats, both eyelids of which were sutured to induce form deprivation myopia, which were kept under poor light conditions for 14 days. Within the same period, animals of Groups 1 and 2 were under natural light conditions. Once 14-day period expired, sutures were taken out from the eyelids. After suture removal, all the animals were performed ultrasound scanning, tonometry to measure IOP, and pachymetry to measure corneal thickness. Ultrasound scanning of rats’ eyes was made using Cinescan (Quantel Medical) with a 20 MHz B probe for anterior eye. The eyelids of the rats were pulled with fingers to drop in a local anesthetic agent. A 10 mm ultrasound gel portion was put on an eye kept open. Thanks to the gel, the probe focus was managed to place in the center of rat’s eye inside a big crystalline lens. An axial B-scanning image clearly visualized contours of the cornea (anterior and posterior contours), anterior chamber, anterior and posterior lens capsule, and vitreoretinal borders. Caliper positioning in a “frozen” image was with a step of 0.1 mm and velocity of 1 550 m/s. The eyeball length was measured along the axial length between the anterior corneal contour and the vitreoretinal border. Maklakov applanation tonometer was used to measure IOP; we used a 2 g plunger, 4 mm in diameter. The procedure was made under topical anesthesia. The corneal thickness in the experimental animals was studied by pachymetry (Handy Pachymetr SP–100, Japan). Data were presented in µm. At two weeks after removal of sutures from the eyelids, the animals were sacrificed under general anesthesia and the eyeballs were enucleated. Objective criterion for myopia development was elongation of anterior-posterior dimension (ARD) of the eyeball which was measured using ultrasound scanning (in vivo) and a digital sliding caliper (Topex) with 0.02 mm accuracy (post mortem). Data obtained were processed using the non-parametric Kruskall-Wallis and Mann-Whitney tests using a software program (Statistica 5.5). Results and Discussion In modern literature there are contradictory reports on a correlation between refractive status and both intraocular pressure and corneal thickness [28–30]. Our data are presented in Table 1 showing that IOP level was significantly increased in the experimental rats with myopia compared with the intact rats. Thus, the rats with form deprivation myopia (Group 2) and in the rats with form deprivation myopia under low light conditions (Group 3) had IOP increased by 12.4 % (р<0.001) and by 29.2% (р< 0.001), respectively, compared with control. In addition, IOP level in Group 3 was significantly different from that in the rats with myopia induced under natural lighting, being higher by 15.0% (р<0.001).
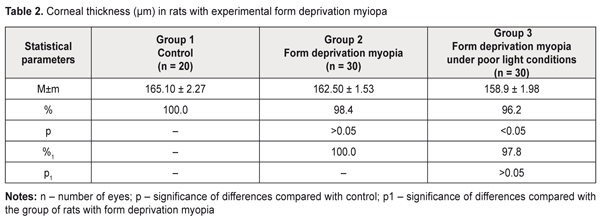
Thus, the data obtained give evidence showing a significant relationship between myopization at an early age and an IOP level in animals. It should be noted that the corneal thickness in the rats with form deprivation myopia under natural lighting almost did not differ from that in the intact animals (Table 2). The form deprivation myopic rats under poor lighting were noted to have corneal thickness loss compared with control, which can be associated with a more pronounced stretching of an outer coat of the eyeball when linear sizes of the eyeball are changed [2,31],
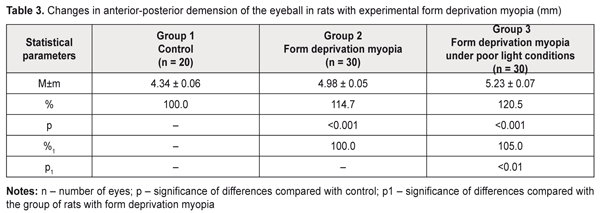
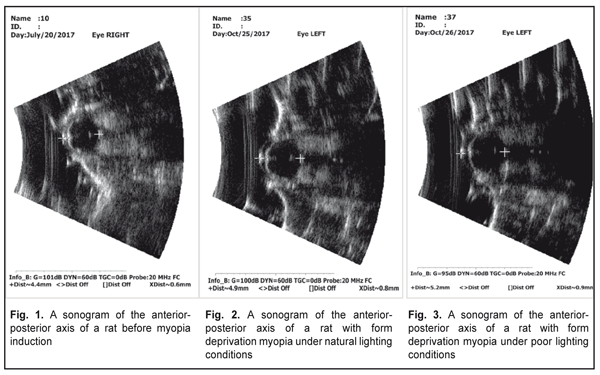
Literature data refer to a possible effect of daylight duration on myopization. It has been shown that eyeball growth under poor light conditions can facilitate axial length elongation [25–27]. Our data on anterior-posterior dimension of the eyeball are given in Table 3, Figures 1, 2, and 3. Thus, this value was significantly increased in the rats with an axial myopia model under poor light conditions: by 21% as compared with the intact animals and by 5% (р<0.001) as compared with the rats with experimental myopia under natural lighting.
Thus, our data on changes in visual organ parameters (intraocular pressure, corneal thickness, anterior-posterior dimension of the eyeball) in young rats when modelling form deprivation myopia under different light conditions give evidence of more rapid myopia progression when lighting is poor. The myopic rats being kept under poor light conditions had significantly more pronounced changes in all studied eyeball parameters involved in myopization compared with those under natural light conditions. Conclusions Form deprivation myopia which is induced in two-week-old rats in the period of eyeball growth by eyelid suture under poor light conditions can be recommended for studying structural and functional characteristics of progressive myopia and developing pathogenetically-oriented treatment methods for the disease. References
|