J.ophthalmol.(Ukraine).2018;2:3-6.
|
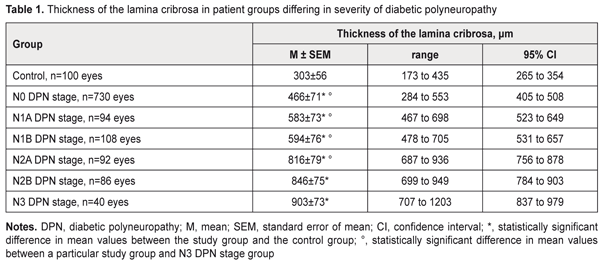
https://doi.org/10.31288/oftalmolzh/2018/2/36 Changes in the lamina cribrosa in patient groups differing in severity of diabetic polyneuropathy M.A. Karliychuk,1 Cand Sc (Med), Assoc. Prof., P.A. Bezditko,2 Dr Sc (Med), Prof. 1 Higher State Educational Establishment of Ukraine «Bukovinian State Medical University»; Chernivtsi (Ukraine) 2 Kharkiv National Medical University; Kharkiv (Ukraine) E-mail: mari13karli@gmail.com TO CITE THIS ARTICLE: Karliychuk MA., Bezditko PA. Changes in the lamina cribrosa in patient groups differing in severity of diabetic polyneuropathy. J.ophthalmol.(Ukraine).2018;2:3-6. https://doi.org/10.31288/oftalmolzh/2018/2/36 Background: To our knowledge, no clinical study has previously been performed to investigate morphometric changes in the lamina cribrosa in diabetes mellitus (DM) patients differing in severity of diabetic polyneuropathy (DPN). Purpose: To identify changes in the lamina cribrosa in patients with DPN differing in severity of the disease. Materials and Methods: A total of 575 patients (1150 eyes) with type 2 diabetes mellitus were included in the prospective analysis of changes in the lamina cribrosa. DPN was diagnosed in 210 (36.5%) patients with DM. In addition to routine eye examination, retinal and optic nerve OCT was performed. Thickness of the cribriform plate and scleral canal area were measured. Results: A relationship was found between the state of the lamina cribrosa and the severity of diabetic polyneuropathy in patients with diabetes mellitus. The mean thicknesses of the lamina cribrosa in DM patients without evidence of DPN, and those with N1A, N1B, N2A, N2B, and N3 stages DPN, were 1.5-fold, 1.9-fold, 2.0-fold, 2.7-fold, 2.8-fold, and 3.0-fold higher than in controls (303 ± 56 μm) (p < 0.001). Conclusion: We found that, in patients with DM, lamina cribrosa thickness increased with increase in severity of DPN. Keywords: diabetic polyneuropathy, thickness of the lamina cribrosa, scleral canal area Introduction Early diagnosing neurological complications of diabetes mellitus (DM) is a challenge, and therefore, the search for reliable methods for diagnosing diabetic polyneuropathy (DPN) is still of importance [1, 2]. Diabetic optic neuropathy is a manifestation of a systemic injury to the nervous system, polyneuropathy, in DM [3]. That is, theoretically, the severity of diabetic nerve fiber damage in general may be assessed through changes in the optic nerve and vice versa, and diabetic retinopathy may be regarded as a “model” of systemic DPN [3-6]. The literature is, however, scarce on this issue. We have failed to find any reports on the features of optic nerve damage in patients with different severity of DPN. Findings of some studies are indicative of potential optic nerve damage (i.e., primary damage to ganglion cell axons, and damage secondary to alterations in their blood supply or axoplasmatic current) in the lamina cribrosa with diabetic changes [7]. Changes in the biochemical properties of the lamina cribrosa in diabetes have been established in a recent ex vivo study [7]. It is well known that optic nerve head (ONH) biomechanics depends on the anatomical, chemical and physical properties of the lamina cribrosa and affect its capacity to provide morphological and functional support for nerve fibers [8-11]. To our knowledge, no clinical study has previously been performed to investigate morphometric changes in the lamina cribrosa in diabetes mellitus patients differing in severity of DPN. The purpose of this study was to identify changes in the lamina cribrosa in patients with DPN differing in severity of the disease. Materials and Methods A total of 575 patients (1150 eyes) with type 2 diabetes mellitus (T2DM) were included in the prospective analysis of changes in the lamina cribrosa. Inclusion criteria were no history of glaucoma; Goldmann IOP of 21 mmHg or less; emmetropic, hypermetropic or low myopic eyes; no cataract or mild age-related cataract; and no history of surgery or laser. The patients’ age ranged from 44 to 69 years (mean ± SD, 55.9±7.8 years). Four hundred and thirteen (71.8%), 93 (16.2%) and 69 (12.0%) had duration of DM of <5, 5-10 and >10 years, respectively. Three hundred and sixty-five patients with DM (63.5%) were classified as those with no evidence for DPN (N0 stage as per the Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy [12, 13]). DPN was diagnosed in 210 (36.5%) patients with DM. Asymptomatic DPN was observed in 101 (17.5%) patients with DM, including 47 (8.1%) patients with N1A stage and 54 (9.4%) patients with N1B stage. Symptomatic DPN was found in 89 (15.5%) patients with DM, including 46 (8.0%) patients with N2A stage and 43 (7.5%) patients with N2B stage, and disabling DPN (N3 stage) was found in 20 (3.5%) patients with DM. The control group comprised 50 healthy individuals (50 eyes). All patients underwent a routine eye examination including visual acuity and a dilated fundus examination. In addition, retinal and optic nerve OCT (RTVue–100, Optovue, Fremont, CA) was performed. We used a novel technique for measuring the thickness of the cribriform plate with software programs LC_Thickness_programm.m and main_low_noise_filters_programm.m which is based on (1) the adaptive compensation algorithm for the removal of high-rank noise in deep layers of the optic nerve head and improvement in visualization of the posterior margin of the cribriform plate and (2) B-scan image processing with three digital filters (a Butterworth low-pass filter for inverse image and Daubechies low-pass wavelet filters for original and inverse images) [14]. In addition, SD-OCT-based measurements of the scleral canal area were performed. LC_cut_position_programm.m was used to select the depth dimension, and LC_diameter_calculation_programm.m was used to improve the selected image with basic digital filters and to identify the highest quality image for measurements [14]. Means (M), standard deviations (σ), standard errors of the mean (SEM) were calculated using Microsoft Excell 2000. In addition, coefficients of variation (Сν), significance (p), 95% confidence intervals (CI) were calculated. Statistical analyses were performed using ANOVA. The level of significance p ≤ 0.05 was assumed. Results A correlation was found between the status of the lamina cribrosa and the severity of DPN in patients with DM. The thickness of the lamina cribrosa was found to depend on the severity of DPN. Thus, in DM patients without evidence of DPN, the mean thickness of the lamina cribrosa was 466±71 μm (range, 284 μm to 553 μm; 95% CI, 405 μm to 508 μm), compared to 583±73 μm (range, 467 μm to 698 μm; 95% CI, 523 μm to 649 μm), 594±76 μm (range, 478 μm to 705 μm; 95% CI, 531 μm to 657 μm), 816±79 μm (range, 687 μm to 936 μm; 95% CI, 756 μm to 878 μm), 846±75 μm (range, 699 μm to 949 μm; 95% CI, 784 μm to 903 μm), and 903±73 μm (range, 707 μm to 1203 μm; 95% CI, 837 μm to 979 μm) in those with N1A, N1B, N2A, N2B, and N3 stages DPN, respectively (Table 1). Therefore, the mean thicknesses of the lamina cribrosa in DM patients without evidence of DPN, and those with N1A, N1B, N2A, N2B, and N3 stages DPN, were 1.5-fold, 1.9-fold, 2.0-fold, 2.7-fold, 2.8-fold, and 3.0-fold higher than in controls (303 ± 56 μm) (p < 0.001). The mean thicknesses of the lamina cribrosa was the highest in DM patients with N3 stage DPN, which was 1.9-fold, 1.5-fold, 1.5-fold, and 1.1-fold higher than in DM patients without evidence of DPN, and those with N1A, N1B, and N2A stages DPN, respectively (р < 0.001), and was not significantly different from that in DM patients with N2A stage DPN (p > 0.05).
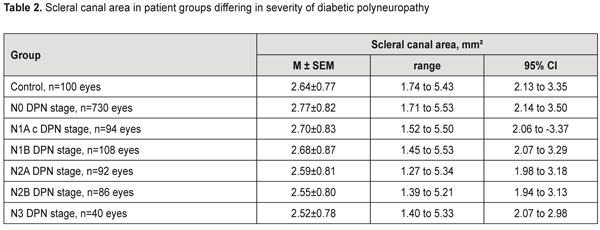
No relationship between the scleral canal area and the severity of the disease was found in patients with DPN. Thus, in DM patients without evidence of DPN, the mean scleral canal area was 2.77±0.82 mm2 (range, 1.71 mm2 to 5.53 mm2; 95% CI, 2.14 mm2 to 3.50 mm2), compared to 2.70±0.83 mm2 (range, 1.52 mm2 to 5.50 mm2; 95% CI, 2.06 mm2 to 3.37 mm2), 2.68±0.87 mm2 (range, 1.45 mm2 to 5.53 mm2; 95% CI, 2.07 mm2 to 3.29 mm2), 2.59±0.81 mm2 (range, 1.27 mm2 to 5.34 mm2; 95% CI, 1.98 mm2 to 3.18 mm2), 2.55±0.80 mm2 (range, 1.39 mm2 to 5.21 mm2; 95% CI, 1.94 mm2 to 3.13 mm2), and 2.52±0.78 mm2 (range, 1.40 mm2 to 5.53 mm2; 95% CI, 2.07 mm2 to 2.98 mm2), in those with N1A, N1B, N2A, N2B, and N3 stages DPN, respectively (Table 2). Therefore, in DM patients without evidence of DPN, and in those with N1A, N1B, N2A, N2B, and N3 stages DPN, the mean scleral canal area was not significantly different from that in the control group (2.64±0.77 mm²) (p > 0.05).
Given the close contact between the lamina cribrosa and retinal ganglion cell axons, it seems reasonable to assume that the thickness of the lamina cribrosa is associated with the course of diabetic optic neuropathy. We found that, in patients with DM, lamina cribrosa thickness increased with increase in severity of DPN. Conclusion
A relationship was found between the state of the lamina cribrosa and the severity of diabetic polyneuropathy in patients with diabetes mellitus, i.e., lamina cribrosa thickness increased with increase in severity of DPN: the mean thicknesses of the lamina cribrosa in DM patients without evidence of DPN, and those with N1A, N1B, N2A, N2B, and N3 stages DPN, were 1.5-fold, 1.9-fold, 2.0-fold, 2.7-fold, 2.8-fold, and 3.0-fold higher than in age-matched controls. References
|