J.ophthalmol.(Ukraine).2018;1:36-42.
|
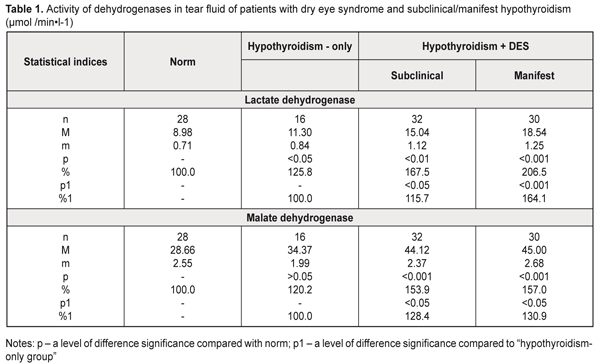
https://doi.org/10.31288/oftalmolzh201813642 Study on metabolic status of tear fluid in hypothyroidism patients with dry eye syndrome M.I. Pavlovskii 1, Post Graduate Student; G.I. Drozhzhyna 2, Dr. Med. Sc., Prof. 1 Lvov regional clinical hospital; Lvov (Ukraine) 2 Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine Odessa (Ukraine) E-mail: cornea@te.net.ua TO CITE THIS ARTICLE: Pavlovskii MI, Drozhzhyna GI. Study on metabolic status of tear fluid in hypothyroidism patients with dry eye syndrome. J.ophthalmol.(Ukraine).2018;1:36-42. https://doi.org/10.31288/oftalmolzh201813642 Introduction. Dry eye syndrome (DES) is associated with a complicated multifactorial mechanism, the peculiarities of which are being studied at the moment. Data on tear production disorders in hypothyroidism and on disturbances in activity of enzymes providing homeostasis of reduced glutathione in DES underline the need to study metabolic alterations in lacrimal gland function in patients with hypothyroidism. Purpose. To study the character of metabolic alterations in the tear fluid in primary hypothyroidism patients with DES. Material and Methods. We examined 78 patients with primary hypothyroidism. Among the patients, there were 16 subclinical hypothyroidism patients without DES (hypothyroidism-only group) and 62 patients with DES (hypothyroidism+DES group) (hypothyroidism type: subclinical, 32 patients; manifest, 30 patients; hypothyroidism compensation: compensation, 28 patients; subcompensation, 20 patients; decompensation, 14 patients). 16 healthy volunteers served as controls. Results. Hypothyroidism patients had more pronounced biochemical changes in the tear fluid in manifest hypothyroidism with DES and decompensated hypothyroidism: the increased activity of acid phosphatase, lactate dehydrogenase, and malate dehydrogenase; disorders in the thiol status against the background of the increased malondialdehyde level. Conclusions. The increased activity of dehydrogenases and acid phosphatase as well as of malondialdehyde against the background of the decreased thiol status in the tear fluid can testify to destruction in cells and subcellular structures of the corneal epithelium in hypothyroidism accompanied with DES that is caused by the action of both lipid peroxidation products and lysosomal enzymes. Key-words: hypothyroidism, dry eye syndrome, tear fluid, dehydrogenase, acid phosphatase, thiol status, lipid peroxidation Introduction It is commonly known that thyroid hormones are primarily responsible for regulation of metabolism and tissue respiration. Experimental studies (Brent GA, 1994; Sarandol E et al, 2005) have shown that the thyroid hormones regulate oxidative metabolism and that hypothyroidism is accompanied with increased oxidative stress in animals [1, 2]. A decreased level of the thyroid hormones in the body is known to lead to hypothyroidism and is accompanied with metabolic, functional, and structural changes in various organs and tissues including the eye [3]. Dias AC and colleagues (2007) have revealed structural and biochemical changes in the tear fluid and conjunctival epithelium on a model of experimental hypothyroidism. However, it is not completely clear how thyroid hormones work on the ocular surface tissues [4]. Our studies in experimental hypothyroidism have revealed metabolic alterations, including those in parameters of oxidative stress in ocular surface tissues, especially in the cornea and tear fluid [5-7]. An important role in pathogenesis of inflammatory and degenerative diseases, in particular eye diseases, belongs to destructive processes caused by oxidative stress products and, consequently, by disorders in lysosomal membrane stability with a release of hydrolyzing enzymes: cathepsins, acid phosphatase, and others [7-10]. However, we have found no data on a state of oxidative stress in ocular surface tissues in patients with hypothyroidism. Dry eye syndrome (DES) is associated with a complicated multifactorial mechanism, the peculiarities of which are being studied at the moment [11, 12]. Data on tear production disorders in hypothyroidism and on disturbances in activity of enzymes providing homeostasis of reduced glutathione in DES [13] underline the need to study metabolic changes in lacrimal gland function in patients with hypothyroidism. Purpose. To study the character of metabolic alterations in the tear fluid in primary hypothyroidism patients with DES. Material and Methods We examined 78 patients with primary hypothyroidism. All patients were followed-up at Lviv Regional State Clinical Diagnostic and Treatment Endocrinologic Center. Among the patients, there were 16 subclinical hypothyroidism patients without DES (hypothyroidism-only group) and 62 patients with DES (hypothyroidism+DES group) (hypothyroidism type: subclinical, 32 patients; manifest, 30 patients; hypothyroidism compensation: compensation, 28 patients; subcompensation, 20 patients; decompensation, 14 patients). 16 healthy volunteers served as controls. The tear fluid was collected using a folded slip of filter paper Filtrak, 5 mm in width and 40 mm in length, which was placed between an eyelid and the cornea of a patient and left for one minute [14]. On moisture treatment, the tear fluid was eluted from the slip in the normal saline solution and used for biochemical studies. In the tear fluid, we determined levels of malondialdehyde [15], oxidized (GSSG) and reduced glutathione (GSH) [16], the activity of malate dehydrogenase [17], lactate dehydrogenase [18] and acid phosphatase [19]. The data obtained were processed using SPSS 11.0 package software [20]. Results Studies on the activity of dehydrogenases in the tear fluid in the hypothyroidism patients showed that hypothyroidism without DES was accompanied by the increased activity of lactate dehydrogenase and malate dehydrogenase by 25.8% (р<0.05) and 20.2% (р<0.05) , respectively, as compared to norm (Table 1). In the patients with subclinical hypothyroidism and DES, the activity of lactate dehydrogenase and malate dehydrogenase significantly increased as compared to norm (р<0.01), by 67.5% and 53.9%, respectively. Herewith, the activity of the studied enzymes significantly differed from the corresponded values in the hypothyroidism-only patients.
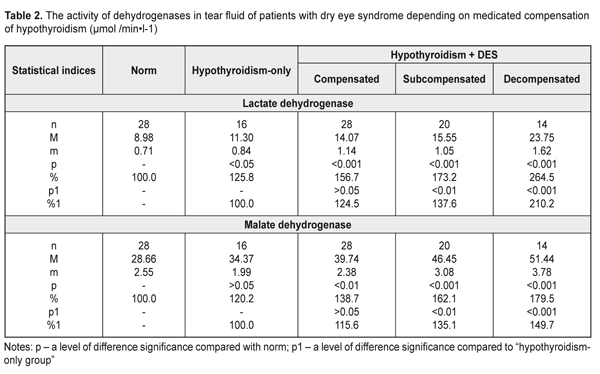
The activity of lactate dehydrogenase and malate dehydrogenase in the tear fluid of the patients with manifest hypothyroidism and DES was increased 2.1 times and 1.6 times, respectively, compared with norm (р<0.001). Comparison of these values with those of the hypothyroidism-only patients showed a significant increase in the dehydrogenase activity in the tear fluid in the patients with manifest hypothyroidism and DES: by 64.1% (р<0.001) and 30.9% (р<0.05) for lactate dehydrogenase and malate dehydrogenase, respectively. With regard to hypothyroidism compensation (Table 2), the lactate dehydrogenase activity in the tear fluid in the patients with hypothyroidism and DES was increased by 56.7%, 73.2%, and 164.5% in compensated, subcompensated, and decompensated hypothyroidism, respectively, compared to norm (р<0.001).
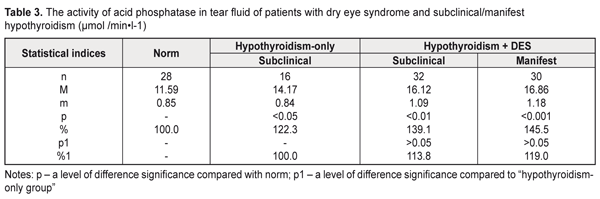
Comparing these data with those in the hypothyroidism-only group, we noted the increased level in the lactate dehydrogenase activity in the tear fluid of the hypothyroidism and DES patients by 24,5% (р>0.05), 37,6% (р<0.01), and 110.2% (р<0.001) for compensated, subcompensated, and decompensated hypothyroidism, respectively. Similar changes were revealed when studying the malate dehydrogenase activity in the tear fluid of the patients with hypothyroidism and DES depending on the hypothyroidism compensation: in compensated hypothyroidism we noted the increased enzyme activity by 38.7% (р<0.01) compared with norm and by 15.6% (р>0.05) compared with the hypothyroidism-only patients; in subcompensated hypothyroidism the enzyme activity was increased by 62.1% (р<0.001) compared with norm and by 35.1% (р>0.01) compared with the hypothyroidism-only patients; in decompensated hypothyroidism the enzyme activity was increased by 79.5% (р<0.001) compared with norm and by 49.7% (р>0.01) compared with the hypothyroidism-only patients (Table 2). Thus, the increased activity of dehydrogenases in the tear fluid in the patients with hypothyroidism and DES gives the evidence of partial destruction of the cell membranes of the corneal epithelium and conjunctiva in this disease. Assessing the activity of acid phosphatase in the tear fluid of the hypothyroidism patients, the attention should be given to a significant increase in the activity both in the presence of DES (by 45.5% and by 39.1% for manifest and subclinical hypothyroidism, respectively), and without DES (by 22.3%) compared with norm (Table 3). As for the hypothyroidism-only patients, changes in the acid phosphatase activity were insufficient: by 13.8% and 19.0% (р>0.05) for subclinical and manifest hypothyroidism.
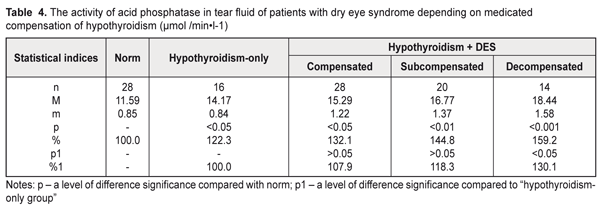
With regard to hypothyroidism compensation (Table 4), the acid phosphatase activity in the tear fluid in the patients with hypothyroidism and DES was increased by 32.1% (р<0.05), 44.8% (р<0.01), and 59.2% (р<0.001) in compensated, subcompensated, and decompensated hypothyroidism, respectively, compared to norm. Comparing with the hypothyroidism-only group, a significant difference was revealed only in decompensated hypothyroidism where the acid phosphatase activity was increased by 30.1% (р<0.05).
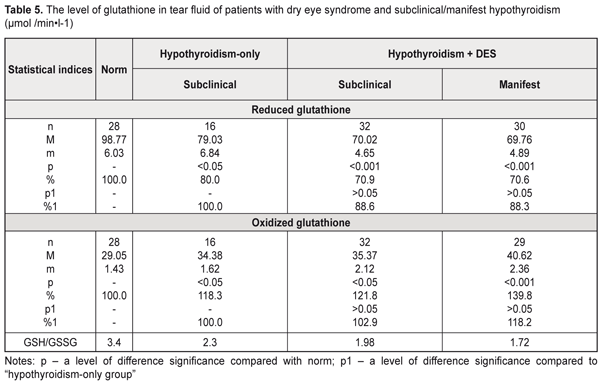
The altered activity of the enzyme in the tear fluid of these patients give the evidence of possible labilization of lysosomal membrane structures in anterior eye tissues, which, in turn, can cause partial destruction of cells and subcellular structures, especially in the corneal epithelium and conjunctiva. The following stage of the study was assessment of a state of lipid peroxidation and antioxidant status in the tear fluid. In this regard, we studied levels of oxidized and reduced glutathione, which is the main antioxidant nonenzymatic cell agent, as well as malondialdehyde, which is a lipid peroxidation end product. In the tear fluid of the hypothyroidism-only patients, the reduced glutathione level was notably decreased by 20.0% while the oxidized glutathione level was increased by 18.3% compared with norm (р<0.05) (Table 5). In subclinical and manifest hypothyroidism with DES, the reduced glutathione level was decreased by 29.1% (р<0.001) and 29.4% (р<0.001), respectively while the oxidized glutathione level was increased by 21.8% (р<0.05) and 39.8% (р<0.001), respectively, compared to norm but not differing significantly from the corresponding values of the hypothyroidism-only patients.
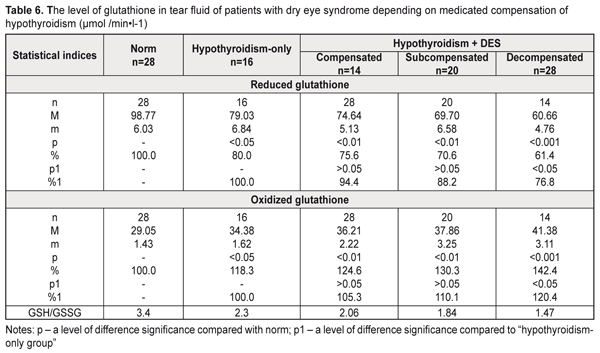
The hypothyroidism patients with DES had a more expressed decrease in the reduced glutathione level and an increase in the oxidized glutathione level in the tear fluid in decompensated hypothyroidism compared to the hypothyroidism-only patients (Table 6). Thus, the hypothyroidism and DES patients had the reduced glutathione patients decreased by 24.2% (р<0.01), by 29.4% (р<0.01), and by 38.6% (р<0.001) in compensated, subcompensated, and decompensated hypothyroidism, respectively, while the oxidized glutathione level was increased by 24.6% (р<0.01), by 30.6% (р<0.01), and by 42.4% (р<0.001) in compensated, subcompensated, and decompensated hypothyroidism, respectively, compared to norm. Comparison of these data with the corresponding values of the hypothyroidism-only patients showed that only in case of decompensated hypothyroidism the reduced glutathione level was decreased by 23.2% (р<0.05) and the oxidized glutathione level was increased by 20.4% (р<0.05). The assessment of the thiol status of the tear was performed as a ratio of reduced to oxidized glutathione (GSH/GSSG). It should be noted that we revealed a significant violation of the thiol status of the tear fluid in the hypothyroidism patients both with and without DES, it was especially pronounced in manifest and decompensated hypothyroidism (Tables 5, 6).
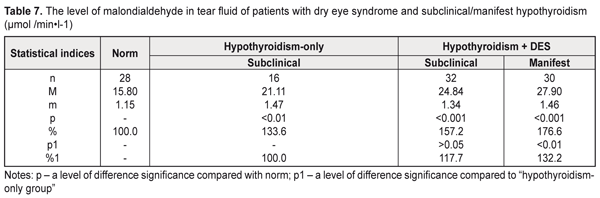
The decreased thiol status in the tear fluid of the examined patients can testify, on the one hand, to intensive consumption of reduced glutathione for detoxification, which leads to glutathione deficiency, and, on the other hand, to possible disorders in resynthesis of the thiol form of glutathione in the anterior eye tissues in hypothyroidism and DES. The decreased thiol status in the hypothyroidism-only patients was accompanied by a significant increase in the malondialdehyde level in the tear fluid: by 33.6% compared with norm (Table 7). The patients with hypothyroidism and DES had a jump in the malondialdehyde level in the tear fluid, compared to norm: 1.8 times and 1.6 times in manifest and subclinical hypothyroidism, respectively. Comparing with the hypothyroidism-only group, significant differences were noted in manifest hypothyroidism: the malondialdehyde level in the tear fluid was increased by 32.2% (р<0.05).
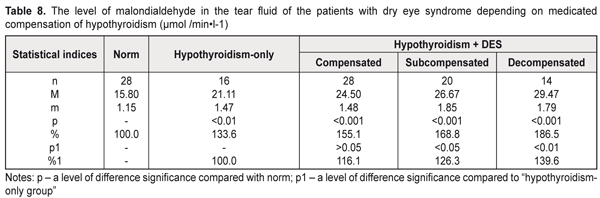
Assessing the malondialdehyde level in the tear fluid of the hypothyroidism patients with DES depending on hypothyroidism compensation, we noted a significant increase in the malondialdehyde level compared to norm (р<0.001): by 55.1%, 68.8%, and 86.5% in compensated, subcompensated, and decompensated hypothyroidism, respectively (Table 8). Compared to the hypothyroidism-only patients, a significant difference was noted both in decompensated (a decrease by 39.6%) and in subcompensated hypothyroidism (a decrease by 26.3%).
Considering the fact that malondialdehyde is the end product of lipid peroxidation, the increased malondialdehyde levels in the tear fluid of the patients with hypothyroidism and DES testify to activated peroxidation and oxidation processes in the anterior eye tissues, which contributes to further development of destructive processes in the cornea and conjunctiva. Assessing in general the state of the metabolic status of the tear fluid of the hypothyroidism patients, it should be noted that the more pronounced biochemical violations were detected in manifest hypothyroidism with DES and in decompensated hypothyroidism. The revealed increase in the acid phosphatase activity in the tear fluid of the hypothyroidism patients with/without DES can cause labilization of cell membranes in the anterior eye tissues which leads to partial destruction of the cells and subcellular structures, especially in the corneal epithelium. Besides, the thiol status disorders against the increased malondialdehyde levels in the tear fluid of the hypothyroidism patients with/without DES give the evidence that in hypothyroidism the ocular surface tissues are under oxidative stress conditions. In this regard, the action of both lipid peroxidation products and lysosomal enzymes can induce further destruction of corneal epithelium cells and subcellular structures which significantly increases the activity of lactate and malate dehydrogenase in the tear fluid of the hypothyroidism patients. The data obtained revealed the patochemical links of pathogenesis of hypothyroidism combined with DES, which can be of a great importance for developing new approaches to treatment of this pathology in order to enhance treatment efficacy. Conclusions First, the more pronounced increase in the activity of lactate and malate dehydrogenase in the tear fluid of the hypothyroidism patients was in manifest hypothyroidism with DES (by 64.1% and 30.9%, respectively) and in decompensated hypothyroidism with DES (by 110.2% and 49.7%, respectively) compared with the hypothyroidism-only patients, which gives the evidence of the activated destructive processes in the ocular tissues. Second, we noted in the tear fluid a significant increase in the activity of acid phosphatase, which is a marker enzyme of cell membrane integrity violation in the anterior eye tissues: by 45.5% in the patients with manifest hypothyroidism and DES compared with norm; by 59.2% in the patients with decompensated hypothyroidism with DES compared with norm; and by 30.1% in the patients with decompensated hypothyroidism with DES compared with the hypothyroidism-only patients. Third, significant violations of the thiol status were revealed in the tear fluid of the patients with hypothyroidism and DES, which testifies to a decreased level of antioxidant protection: in manifest hypothyroidism, the reduced glutathione level was decreased by 29.4% and the oxidized glutathione level was increased by 39.8%, compared to norm; in decompenstaed hypothyroidism, the reduced glutathione level was decreased by 23.2% and the oxidized glutathione level was increased by 20.4%, compared to the hypothyroidism-only patients.
Fourth, the malondialdehyde level in the tear fluid was increased by 32.2% in manifest hypothyroidism with DES and by 39.6% in decompensated hypothyroidism, compared with the hypothyroidism-only patients, which reflexes activated peroxidation processes in the anterior eye tissues in these patients.
References
|