J.ophthalmol.(Ukraine).2018;1:13-18.
|
https://doi.org/10.31288/oftalmolzh201811318 Comparison of morphometric changes in the chorioretinal complex after anti-VEGF treatment in patients with pathologic myopia versus those with neovascular age-related macular degeneration O.M. Blavatska 1, T.B. Kustryn 2, A.R. Korol 2, Dr Sc (Med) 1 Danylo Halytsky Lviv National Medical University; Lviv (Ukraine) 2 Filatov Institute of Eye Diseases and Tissue Therapy; Odessa (Ukraine) E-mail: syannya@rambler.ru, laserfilatova@gmail.com TO CITE THIS ARTICLE: Blavatska OM, Kustryn TB, Korol AR. Comparison of morphometric changes in the chorioretinal complex after anti-VEGF treatment in patients with pathologic myopia versus those with neovascular age-related macular degeneration. J.ophthalmol.(Ukraine).2018;1:13-8. https://doi.org/10.31288/oftalmolzh201811318
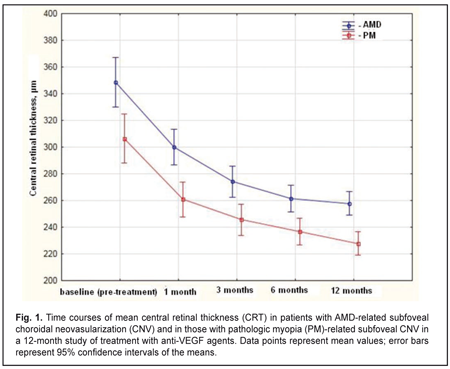
Purpose: To compare morphometric changes in the chorioretinal complex after anti-vascular endothelial growth factor (anti-VEGF) treatment in patients with pathologic myopia (PM)-related choroidal neovascularization (CNV) versus those with age-related macular degeneration (AMD)-related CNV. Materials and Methods: This was a prospective comparative uncontrolled cohort study involving 156 patients (169 eyes) with subfoveal CNV. Of these, 82 (85 eyes) had pathologic PM-related CNV, and 74 (85 eyes) had AMD-related CNV. Patients with AMD underwent a treatment regimen of three mandatory monthly 0.5-mg (0.05-mL) intravitreal ranibizumab injections followed by pro re nata (PRN) dosing according to re-treatment criteria. Patients with PM underwent a treatment regimen of two mandatory monthly 0.5-mg (0.05-mL) intravitreal ranibizumab injections or 2-mg (0.05-mL) intravitreal aflibercept injections followed by PRN dosing according to re-treatment criteria. Primary outcomes were central retinal thickness (CRT) and CNV thickness at month 12. Secondary outcomes included best-corrected visual acuity (BCVA) and number of injections at month 12. Results: At month 12, (1) mean OCT-based CRT in AMD and PM groups decreased from 349 μm to 258 μm (P = 0.00), and from 306μm to 228 μm (P = 0.00), respectively, and (2) mean CNV thickness in AMD and PM groups decreased from 183 μm to 108 μm (P = 0.00), and from 229 μm to 173 μm (P = 0.00), respectively. In addition, mean (SD) BCVA in the AMD group improved from 0.25 (0.23) at baseline to 0.33 (0.29), whereas that in the PM group improved from 0.17 (0.11) to 0.35 (0.19). The mean number of anti-VEGF injections was 5.4 (2.1) and 2.2 (0.6) for the AMD and PM groups, respectively, with the between-group difference being statistically significant (P = 0.00). Conclusions: Decrease in central retinal thickness and improvements in visual acuity after anti-VEGF treatment for AMD-related subfoveal CNV were similar to those after anti-VEGF treatment for PM-related subfoveal CNV. At month 12, percent decreases in CNV thickness compared to baseline were 26% and 41% for anti-VEGF treated patients with AMD-related and PM-related subfoveal CNV, respectively. Patients with AMD-related CNV required, on average, 3 more anti-VEGF injections than those with PM-related CNV. Keywords: pathologic myopia, age-related macular degeneration, choroidal neovascularization, anti-VEGF agents Introduction Choroidal neovascularization (CNV) is one of the most common complications in age-related macular degeneration (AMD) and pathologic myopia (PM) [1-3]. Composition of CNV includes fibrovascular tissue that develops after damage to Bruch’s membrane with subsequent ingrowth of choroidal vessels beneath the retina. Subfoveal CNV has an especially unfavorable prognosis as it is in this condition that abrupt and irreversible loss of central vision occurs in most patients despite treatment [4]. Laser photocoagulation, transpupillary thermotherapy and photodynamic therapy have been proposed for the treatment of CNV [5-7]. Currently, intravitreal anti-VEGF injections are considered by many to be the optimal treatment approach for CNV. Anti-VEGF agents like ranibizumab and aflibercept are widely used to treat CNV in AMD and PM [8-14]. Efficacy and safety of ranibizumab for the treatment of CNV in AMD have been demonstrated in the MARINA [11], ANCHOR [8], and PIER [10] clinical trials involving 716, 423 and 184 patients, respectively, whereas those of aflibercept for the treatment of subfoveal CNV lesions secondary to wet AMD, in the VIEW 1 and VIEW 2 [9] clinical trials involving 1217 and 1240 patients, respectively. Efficacy and safety of ranibizumab for the treatment of myopic CNV have been investigated in the RADIANCE [13] and REPAIR [12] clinical trials in 277 and 65 patients, respectively, whereas those of aflibercept for the treatment of myopic choroidal neovascularization, in the MYRROR [14] clinical trial in 122 patients. In the previous study of ranibizumab versus aflibercept in patients with CNV in AMD, we have found that anatomic and visual outcomes were similar among the treatment groups at 12 months [15]. The purpose of the present study was to compare anti-VEGF agent-induced changes in morphometric characteristics of the chorioretinal complex between patients with pathologic myopia (PM)-related subfoveal CNV and those with AMD-related subfoveal CNV. Materials and Methods This was a prospective comparative uncontrolled cohort study involving 156 patients (169 eyes) with subfoveal CNV. Of these, 82 (85 eyes) had PM-related CNV, and 74 (85 eyes) had AMD-related CNV. The study was conducted at the Laser Eye Microsurgery Department of the Filatov Institute and at the Department of Ophthalmology, Postgraduate Study Faculty, the Danylo Halytsky Lviv National Medical University, from March 2012 to May 2016. The study followed the ethical standards stated in the Declaration of Helsinki and was approved by the Local Ethics Committee. Patients were informed about the study procedure and provided written informed consent. Inclusion criteria for patients with AMD were classic subfoveal CNV, age ≥50 years, recent diagnosis of CNV associated with AMD (up to 3 months previously), and best-corrected visual acuity (BCVA) of at least 0.05. Inclusion criteria for patients with PM were classic subfoveal CNV; either myopic spherical equivalent ≥ 6 D or myopic macular changes like lacquer cracks, foci of chorioretinal atrophy or posterior staphyloma; axial length ≥ 26.5 mm; a history of pathologic myopia; recent diagnosis of CNV associated with PM (up to 2 months previously); and BCVA of at least 0.05. Patients with AMD underwent a treatment regimen of three mandatory monthly 0.5-mg (0.05-mL) intravitreal ranibizumab injections followed by pro re nata (PRN) dosing according to re-treatment criteria. Patients with PM underwent a treatment regimen of two mandatory monthly 0.5-mg (0.05-mL) intravitreal ranibizumab injections or 2-mg (0.05-mL) intravitreal aflibercept injections followed by PRN dosing according to re-treatment criteria. In both groups, the retreatment criteria included worsening of BCVA, ophthalmoscopic evidence of subretinal hemorrhage, OCT evidence of increased macular thickness or CNV thickness, and fluorescein angiography (FA) evidence of increased macular hyperfluorescence. The injections were given in a standardized manner throughout the study. Study eyes underwent thorough examination including BCVA assessment, biomicroscopy, macular OCT (Stratus 3000, Carl Zeiss Meditec, Jena, Germany), color fundus photography and FA (TRC 50-EX, Topcon, Tokyo, Japan). Primary outcomes were central retinal thickness (CRT) and CNV thickness at month 12. Secondary outcomes included BCVA and number of injections. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. The data are presented as mean ± standard deviation (SD), median and range. Time courses of BCVA, CRT and CNV thickness after initiation of treatment were analyzed with repeated-measures ANOVA. The Mann-Whitney rank test was used for between-group comparisons at each time point. P < 0.05 was considered statistically significant in all the analyses. Results The mean (SD) CRT at baseline was 349 (100) μm and 306 (69) μm in the AMD and PM groups, respectively (P = 0.0). Statistically significant decreases in mean (SD) CRT were observed in both groups over the follow-up period. The between-group differences in mean (SD) CRT at each time point were statistically significant. At month 12, mean OCT-based CRT in AMD and PM groups decreased by 26% (from 349 μm to 258 μm, P = 0.00), and by 25% (from 306 μm to 228 μm , P = 0.00), respectively (Fig. 1). The between-group difference at the final follow-up was statistically significant (P = 0.00).
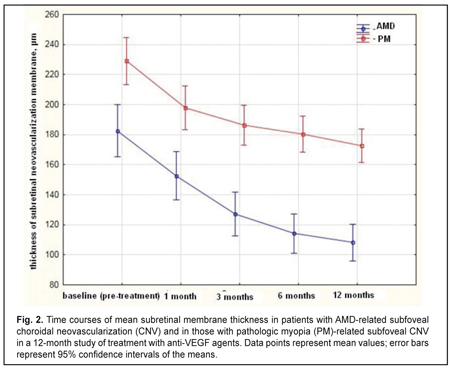
The mean (SD) CNV thickness at baseline was 183 (85) μm and 229 (62) μm in the AMD and PM groups, respectively (P = 0.0). Statistically significant decreases in mean (SD) CNV thickness were observed in both groups over the follow-up period (Fig. 2). The between-group differences in mean (SD) CNV thickness at each time point were statistically significant. Thus, at the final time point, mean CNV thickness in AMD and PM groups decreased by 41% (from 183 μm to 108 μm, P = 0.00), and by 24% (from 229 μm to 173 μm , P = 0.00), respectively. The between-group difference in mean CNV thickness at month 12 was statistically significant (P = 0.02).
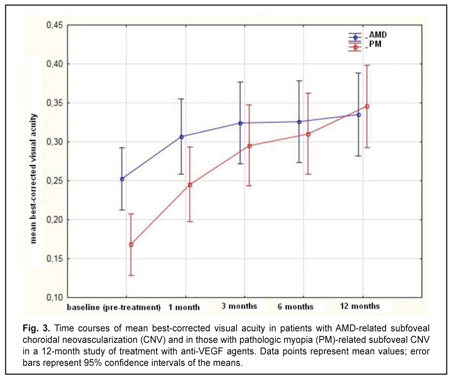
The mean (SD) BCVA at baseline was 0.25 (0.23) and 0.17 (0.11) in the AMD and PM groups, respectively (P = 0.02). The mean (SD) BCVA at month 12 was 0.33 (0.29) (P = 0.00) and 0.35 (0.19) (P = 0.00) in the AMD and PM groups, respectively. The between-group difference in BCVA at month 12 was not statistically significant (P = 0.8). Statistically significant increases in mean (SD) BCVA were observed in both groups over the follow-up period (Fig. 3).
The mean number of anti-VEGF injections was 5.4 (2.1) and 2.2 (0.6) for the AMD and PM groups, respectively, and the between-group difference was statistically significant (P = 0.00). No case of infectious complication, retinal detachment or cardiovascular complication was noted in either group over the follow-up period. Discussion In this 12-month prospective study, we compared morphometric and functional outcomes between patients with AMD-associated CNV and those with PM- associated CNV after treatment with anti-VEGF agents. We found that the mean (SD) CRT at baseline in the AMD and PM groups, respectively, was 349 (100) μm and 306 (69) μm (P = 0.0). Such a between-groups difference is explained by a more pronounced exudation in patients with AMD-associated CNV, given the fact that the CNV thickness was significantly greater in patients with PM-associated CNV than in those with AMD-associated CNV (229 (62) μm versus 183 (85) μm, respectively, P = 0.00). Statistically significant decreases in mean (SD) CRT were observed both in myopic and AMD patients treated with ant-VEGF agents over the follow-up period. At 12 month, the mean (SD) CRT significantly decreased by 91 μm (from 349 μm to 258 μm) in the AMD group and by 78 μm (from 306 μm to 228 μm) in the AMD group. In addition, in our study, the mean number of anti-VEGF injections was 5.4 (2.1) and 2.2 (0.6) for the AMD and PM groups, respectively, with the between-group difference being statistically significant (P = 0.00). In the PIER study, patients with AMD-associated CNV received 3 monthly loading 0.5-mg ranibizumab injection then quarterly injections (corresponding to a total of 6 injections within a year), the mean retinal thickness at the fovea was significantly decreasing over the study period and decreased by 120 μm compared to baseline at month 12 [10]. In the VIEW 1 study, in patients with wet AMD-related choroidal neovascularization lesions scheduled to intravitreal aflibercept 2 mg monthly, the mean CRT was decreasing over 52 weeks similarly to that in the PIER study [9]. It was shown in the RADIANCE study that, in patients with PM-related choroidal neovascularization, the mean CRT decrease at month 12 was −71.3 μm after treatment with two 0.5-mg ranibizumab injections [13]. Cha and co-authors compared the 12-month efficacy of ranibizumab (22 patients) versus bevacizumab (42 patients) for myopic choroidal neovascularization. In their study, central foveal thickness in the ranibizumab group decreased by 22.43% from baseline after 2.43 ± 1.04 injections [16]. The MYRROR study demonstrated that, in patients with myopic choroidal neovascularization, the mean CRT decrease at week 48 was −86.2 μm (from 349.7 μm to 263.5 μm) after treatment with two 2-mg intravitreal aflibercept injections [14]. In our study, the mean (SD) CNV thickness in the AMD and PM groups, respectively, was 183 (85) μm and 229 (62) μm (P = 0.0) at baseline, and decreased by 41% and 24% at the final time point, and these improvements were significant in both groups. In the VIEW 1 study, the mean decrease in choroidal neovascularisation area in patients with AMD-related choroidal neovascularisation treated with 0.5-mg intravitreal ranibizumab injections and those treated with 2-mg intravitreal aflibercept injections, was 4.2 (5.6) μm2 and 4.6 (5.5) μm2, respectively [9]. In patients with myopic CNV, the mean area of fluorescein angiography leakage decreased by 0.31 (1.65) μm2 at month 12 was after treatment with two 0.5-mg intravitreal ranibizumab injections [13]. The MYRROR study demonstrated that, in patients with myopic choroidal neovascularization, the mean decrease in the area of neovascularization was 0.21D of the optic disc after treatment with two 2-mg intravitreal aflibercept injections [14]. We also investigated the time courses of BCVA in AMD and PM. It is noteworthy that statistically significant increases in mean (SD) BCVA were observed in the AMD and PM groups after treatment with anti-VEGF agents. However, the difference in mean BCVA between the AMD and PM groups was significant at baseline (0.25 (0.23) and 0.17 (0.11), respectively, P = 0.02), but not at the final time point (0.33 (0.29) and 0.35 (0.19), respectively, P = 0.8). A number of multicenter clinical trials also reported on improvements in BCVA in patients with AMD-related CNV and in those with PM-related CNV within the course of treatment with anti-VEGF agents [9, 11, 13, 14]. Thus, in the MARINA study, at month 12, the mean improvement in visual acuity was 7.2 letters in patients with wet AMD treated with 0.5-mg intravitreal ranibizumab [11]. The VIEW 1 study demonstrated that patients receiving aflibercept 2 mg monthly for AMD-related CNV, on average gained 10.9 letters by week 52 [9]. In the RADIANCE study, the mean BCVA gain from baseline to month 12 was 13.8 letters for patients with myopic CNV treated with two loading injections of 0.5-mg intravitreal ranibizumab [13]. The MYRROR study demonstrated that, by week 48, the aflibercept 2-mg group of patients with MP-related CNV gained a mean 13.5 letters [14]. A limitation of our study is that visual acuity data are reported in units different from those used in studies by foreign researchers. Another limitation is that we investigated the time course of changes in CNV thickness occurring post anti-VEGF treatment based on OCT data, whereas foreign researchers investigated it based on FA data. In addition, our PM group included patients treated with two anti-VEGF agents, ranibizumab and aflibercept. We believe that such an approach to group composition is viable, as in PM-related CNV, morphological changes induced by treatment with the former agent are similar to those induced by treatment with the later agent [15, 17]. Conclusions
Decrease in central retinal thickness and improvements in visual acuity after anti-VEGF treatment for AMD-related subfoveal CNV were similar to those after anti-VEGF treatment for PM-related subfoveal CNV. At month 12, percent decreases in CNV thickness compared to baseline were 26% and 41% for anti-VEGF treated patients with AMD-related and PM-related subfoveal CNV, respectively. Patients with AMD-related CNV required, on average, 3 more anti-VEGF injections than those with PM-related CNV.
References
|