J.ophthalmol.(Ukraine).2018;1:7-12.
|
https://doi.org/10.31288/oftalmolzh20181712 Predicting the risk for progression of acquired myopia in school-age children T.E. Tsybulska, Cand Sc (Med), N.G. Zavgorodnia, Dr Sc (Med), Prof., O.E. Pashkova, Dr Sc (Med) Zaporizhzhia State Medical University; Medical Centre, VIZUS LLC Zaporizhzhia (Ukraine) E-mail: tamila.eye@gmail.com TO CITE THIS ARTICLE: Tsybulska TE, Zavgorodnia NG, Pashkova OE. Predicting the risk for progression of acquired myopia in school-age children. J.ophthalmol.(Ukraine).2018;1:7-12. https://doi.org/10.31288/oftalmolzh20181712
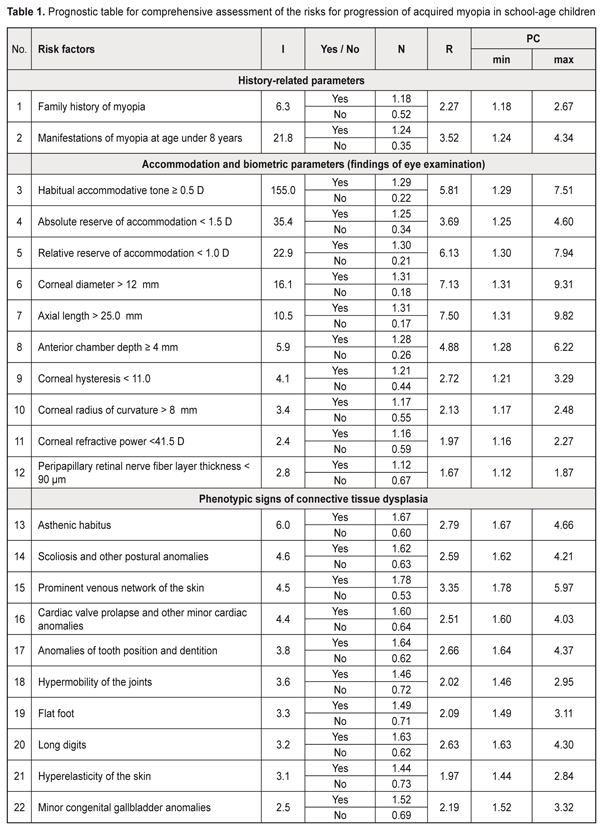
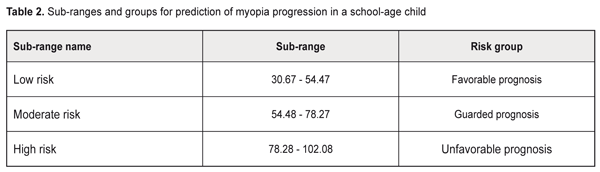
Background: Prediction of myopia progression in children is of practical relevance. Purpose: To develop a prediction table for comprehensive assessment of the risks for progression of acquired myopia in school-age children. Materials and Methods: One hundred and forty-four low myopic children (288 eyes) underwent the examination. Of these, 62 (124 eyes) had progressive myopia, and 82 (164 eyes) had stable myopia. Prediction of the risk of myopia progression in children was performed with the use of the Shigan technique for normalization of strongly intensive measures based on the probabilistic Bayesian approach. Results: Minimal and maximal values for prediction coefficients with regard to the most informative predictors for myopia progression were calculated, and the relevant prediction table was generated. The table included history-, accommodation-, and biometry-related predictors, as well as those related to the phenotypical signs of connective tissue dysplasia (CTD) as follows: history-related predictors: family history of myopia (PCmin, 1.249; PCmax, 4.34) and manifestations of myopia at age under 8 years (PCmin, 1.18; PCmax, 2.67); accommodation-related predictors: habitual accommodative tone ≥ 0.5 D (PCmin, 1.29; PCmax, 7.51), absolute reserve of accommodation < 1.5 D (PCmin, 1.25; PCmax, 4.6), relative reserve of accommodation < 1.0 D (PCmin, 1.3; PCmax, 7.94); ocular biometry-related predictors: corneal diameter > 12 mm (PCmin, 1.31; PCmax, 9.31), axial length of the eye > 25.0 mm (PCmin, 1.31; PCmax, 9.82), anterior chamber depth ≥ 4 mm (PCmin, 1.28; PCmax, 6.22), corneal hysteresis < 11.0 (PCmin, 1.21; PCmax, 3.29), corneal radius of curvature > 8 mm (PCmin, 1.17; PCmax, 2.5), corneal refractive power < 41.5 D (PCmin, 1.17; PCmax, 2.5), peripapillary retinal nerve fiber layer thickness (PCmin, 1.12; PCmax, 1.9), P < 0.05; CTD-related predictors: asthenic habitus (PCmin, 1.67; PCmax, 4.66), scoliosis and other postural anomalies (PCmin, 1.61; PCmax, 4.21), prominent venous network of the skin (PCmin, 1.75; PCmax, 5.97), cardiac valve prolapse and other minor cardiac anomalies (PCmin, 1.6; PCmax, 4.03), anomalies of tooth position and dentition (PCmin, 1.64; PCmax, 4.37), long digits (PCmin, 1.63; PCmax, 4.3), flat foot (PCmin, 1. 49; PCmax, 3.1), hypermobility of the joints (PCmin, 1.46; PCmax, 2.95), hyperelasticity of the skin (PCmin, 1.44; PCmax, 2.84), and congenital gallbladder anomalies (PCmin, 1.52; PCmax, 3.32), P < 0.05. The newly identified range for the risk of progression of myopia was subdivided into 3 equal sub-ranges (low, moderate and high likelihood for the risk, of 30.67-54.47, 54.48-78.27 and 78.28-102.08, respectively). Conclusion: The use of the prognostic table proposed allows easy identification of school-age myopic children at risk for the progression of myopia, with subsequent development of customized diagnosis and treatment plans. Keywords: myopia, prediction, school-age children Introduction Myopia is by far the most common ocular disease in children [1-3]. Early diagnosis and prevention of the progression of acquired myopia is an important area of practice for the ophthalmologist. Pediatric myopia is increasingly considered not as a purely ocular disease, but rather as a multisystem and multifactorial disease that requires effective communication and cooperation among the physicians of various specialties [1, 4-7]. Thirteen to fifty-three percent of the Ukrainian pediatric population have phenotypic manifestations of the syndrome of unspecified connective tissue dysplasia (SUCTD) [5, 6]. Studies by the leading experts in the field have demonstrated that myopia is one of the manifestations of SUCTD [4-6]. Without a doubt, connective tissue dysplasia and other factors related to age, manifestations of the disease, and functional status of the visual system have an impact on the course of myopia in children. Opportunities for the prediction of myopia progression in children, are, therefore, of practical relevance in ophthalmology and pediatrics, and require further investigation. Prognostic criteria, if identified, will allow early identification of myopic children with unfavorable prognosis, with customized treatment planning aimed at delaying the progression of disease and preventing the onset of complications. The purpose of the study was to develop a prediction table for comprehensive assessment of the risks for progression of acquired myopia in school-age children based on the history data, accommodation and biometric parameters of the eye and phenotypic signs of connective tissue dysplasia. Materials and Methods This was a retrospective study of 144 low myopes (288 eyes) aged 7-15 years who were followed for 12 months. They were retrospectively divided into two groups. The progressive myopia (PM) group (62 children, 124 eyes) included children who demonstrated an increase in static refractive error at 12 months, and the stable myopia (SM) group included the rest of the children (82 children, 164 eyes). The examination included (1) thorough history taking, and (2) evaluation of the phenotypic signs of SUCTD through history, medical records and physical examination, and based on specialty opinion (from pediatrics, orthopedics, neuropathology, etc). Sixty-five of the 144 children of the study were found to have multiple phenotypic signs of connective tissue dysplasia (CTD), which allowed for diagnosis of SUCTD in them [4, 5]. Routine eye examination included visual acuity test, accommodation measurement, biomicroscopy, ophthalmoscopy, optical biometry (IOL-Master 500, Carl Zeiss Meditec, Jena, Germany), Ocular Response Analyzer measurements, and Cirrus spectral domain optical coherence tomography (Cirrus HD-OCT 4000 model; Carl Zeiss Meditec). In addition, autorefractometry and keratometry (HRK-7000; HUVITZ, Gunpo, Korea) were performed under cycloplegia. The study included several stages, and its results are presented as a prediction table. At the first stage, the criteria for the development of prediction table were selected. Informativeness of a clinical sign was calculated using the formula below:
I = c1/d1: c2/d2, where c1 is a number of PM-group children having the sign under assessment, c2 is a number of SM-group children having the sign under assessment, d1 is a number of PM-group children having no sign under assessment, and d2 is a number of SM-group children having no sign under assessment. Any clinical sign having an I value of 2.0 or more was considered as a risk factor for progression of myopia and was taken into account when forming the prediction table. Subsequently, prediction of the risk of myopia progression in children was performed with the use of the Shigan technique for normalization of strongly intensive measures based on the probabilistic Bayesian approach [8]. Normalized rate index was calculated using the formula below:
N = m/M, where N is a normalized rate index, m is a relative index of the sign under analysis in children with fast progressive myopia (%), M is a relative index of the sign under analysis in all the examined children with myopia (%). Next, meaningfulness of the factors and respective factor levels were determined with the use of a relative risk index (R) that represents a ratio of a maximum rate index (c) to minimum rate index (c) for a particular factor (R = c/d). R = 1 if the factor has no impact. The higher the value of R, the greater the meaningfulness of the factor for emergence of the pathology. At the last stage, the prediction coefficient (PC) was calculated using the formula below:
PC = N × R, where N is a normalized rate index, R is a relative risk index [8]. Results and Discussion An analysis of 30 potential risk factors for progression of acquired myopia was conducted. These potential risk factors were divided into three categories: anamnestic data, accommodation and biometric parameters of the eye (based on the eye examination), and phenotypic signs of connective tissue dysplasia (CTD). The values of informativeness index (I) were calculated for each factor. The factors with I values less than 2.0 were excluded from further analysis. In this way, we selected 22 potential risk factors for progression of acquired myopia. These factors along with their levels (Yes/ No) and relative risk index (R) values (that depend on the power of the factor) were used to create a prediction table for comprehensive assessment of the risk for progression of acquired myopia in school-age children (Table 1).
The following were identified as the most informative history-, accommodation-, and biometry-related predictors, as well as those related to the phenotypical signs of connective tissue dysplasia (CTD): history-related predictors: family history of myopia and manifestations of myopia at age under 8 years; accommodation-related predictors: habitual accommodative tone ≥ 0.5 D, absolute reserve of accommodation < 1.5 D, and relative reserve of accommodation < 1.0 D; ocular biometry-related predictors: corneal diameter > 12 mm, axial length of the eye > 25.0 mm, anterior chamber depth ≥ 4 mm, corneal hysteresis < 11.0 (among biometric parameters of the eye). In addition, phenotypic signs of CTD (namely, asthenic habitus, scoliosis, postural anomalies, prominent venous network of the skin, cardiac valve prolapse and other minor cardiac anomalies, and anomalies of tooth position and dentition) were found to be important for predicting progression of myopia. Based on the data presented in the prediction table, we identified the likely range of risk values for progression of acquired myopia in school-age children. The initial value for the risk of progression of myopia was calculated as a sum of minimal prediction coefficients: ΣPCmin= 1.18+1.24+1.29+1.25+1.30+1.31+1.31+ 1.28+1.21+1.17+1.12+1.16+1.67+1.62+1.78+1.60+1.64+1.46+1.49+1.63+1.44+1.52=30.67. The maximal value for the risk of progression of myopia was calculated as a sum of maximal prediction coefficients: ΣPCmax= 2.67+4.34+7.51+4.60+7.94+9.31+9.82+ 6.22+3.29+2.48+1.87+2.2+7+4.66+4.21+5.97+4.03+4.37+2.95+3.11+4.30+2.84+3.32=102.08. Therefore, we identified a likely range of risk values, from 30.67 to 102.08, for progression of myopia in school-age children. The greater the sum of prediction coefficients, the more likely is the progression of myopia in the patient. The newly identified range was then subdivided into 3 equal subranges (Table 2): low, moderate and high likelihood for the risk of progression of myopia, of 30.67-54.47, 54.48-78.27 and 78.28-102.08, respectively. That is, the prediction risk of progression of myopia matches the sum of prediction coefficients. Based on these data, patients may be divided into 3 groups with regard to prediction of individual prognosis for further progression of myopic refractive error: favorable prognosis, guarded prognosis and unfavorable prognosis.
Clinical example Patient K., a 10-year old boy, diagnosed with bilateral low myopia Right eye (OD): uncorrected visual acuity (UCVA), 0.2; visual acuity with correction –2.5 D. sph., 0.2; Left eye (OS): UCVA, 0.1; visual acuity with correction –3.0 D. sph., 1.0, History-related risk factors: family history of myopia (+2.67), manifestations of myopia at age 7 years (+4.34). Accommodation--related risk factors: habitual accommodative tone, OD=1.0 D, OS =1.0 D (+7.51); absolute reserve of accommodation, OD=0.5 D, OS =0.5 D (+4.6), and relative reserve of accommodation, OD=0.5 D, OS =0.5 D (+7.94); Ocular biometrics-related risk factors: corneal diameter > 12 mm, OD=12.8 mm, OS=12.7 mm (+9.31); axial length of the eye > 25.0 mm, OD=25.5 mm, OS=25.6 mm (+9.82); anterior chamber depth ≥ 4 mm, OD=4.0 mm, OS=4.0 mm (+6.22); corneal hysteresis < 11.0; OD=10.6, OS=10.3 (+3.29); corneal radius of curvature, OD= 8.2 mm, OS=8.2 mm (+2.48); corneal refractive power, OD= 40.5 D, OS=40.5 D (+2.27); peripapillary retinal nerve fiber layer thickness, OD=90 μm, OS=87 μm (+1.87). The findings related to phenotypic signs of CTD were as follows: The patient had an asthenic habitus (+4.66) and scoliosis (+4.21). The venous network of the skin was prominent (+5.97). Cardiac valve prolapse and other minor cardiac anomalies (namely, a false cord running across the left cardiac ventricle) were revealed (+4.03). Anomalies of tooth position and dentition were not found (+0.62). Hypermobility of the joints was present (+2.95). Flat foot were noted (+3.11), but neither long digits (+0.62) nor hyperelasticity of the skin (+0.73) were seen. Congenital gallbladder anomalies were found (+3.32). Therefore, the sum of the prediction coefficients was ΣPC=2.67+4.34+7.51+4.6+7.94+9.31+9.82+6.22++3.29+2.48+2.27+1.87+4.66+4.21+5.97+4.03+0.62+ 2.95+3.11+0.62+0.73+3.32=92.54 Conclusion: the patient was at high risk for progression of his acquired myopia (an unfavorable prognosis). At re-examination 12 months later, a substantial progression of myopia was noted. OD: UCVA, 0.09; visual acuity with correction –3.5 D. sph., 1.0; axial length, 26.6 mm; OS: UCVA, 0.09; visual acuity with correction –3.75 D. sph., 1.0; axial length, 26.6 mm. It should be noted that predicting myopia progression in myopic children is of special importance in practical ophthalmology. Avetisov, Bushuieva, Boichuk and co-authors, Svirin, Iomdina, Tarutta and others have contributed to the development of prediction approaches related to the progression of myopic refractive error [1, 2, 9, 10]. Most of these authors give a priority to such prediction criteria as anatomical and optical parameters of the patient’s eye, early onset of disease, poor accommodative capacity, refractive error at the time of examination, and decreased peripapillary retinal nerve fiber layer thickness [1, 2, 9]. Several studies have reported on the impact of pediatric extraocular pathology on the progression of myopia. Thus, in the study by Chetyz [7], an extraocular pathology was found in 96.6% of myopic children of different age groups and in only 31% of non-myopic children. Myopia is an important marker of CTD that contributes to the development of a somatic disease in children [4-6]. Several studies reported on evidence of progression of myopia in children with flat foot, scoliosis, and/or hypermobility of the joints [1, 10]. However, to the best of our knowledge, there have been no reports on the comprehensive assessment of accommodation and biometric parameters of the eye and their associations with phenotypical signs of unspecified CTD regarding the progression of myopia in children with acquired disease. There is no general consensus on which phenotypical markers of unspecified CTD contribute most to the risk of myopia progression in children with acquired myopia. Our findings evidence the following: it is children with unspecified CTD who are at high risk of myopia progression, which underlines the importance of (a) a comprehensive approach to examination of children with the disease and (b) close collaboration of the pediatric ophthalmologist and pediatrician or family doctor. Conclusion First, we investigated risk factors for progression of acquired myopia in school-age children. The results of this investigation obtained with the use of the prediction table demonstrated (a) meaningfullness of such factors as corneal diameter > 12 mm, axial length of the eye > 25.0 mm, anterior chamber depth ≥ 4 mm, accommodative impairments, and early onset and family history of disease. In addition, phenotypic signs of CTD (namely, asthenic habitus, scoliosis, postural anomalies, prominent venous network of the skin, cardiac valve prolapse and other minor cardiac anomalies, and anomalies of tooth position and dentition) were found to be important for predicting progression of myopia.
Second, the use of the prognostic table proposed allows easy identification of school-age children at risk for the progression of myopia, with subsequent development of customized diagnosis and treatment plans.
References
|