J.ophthalmol.(Ukraine).2018;1:67-73.
|
https://doi.org/10.31288/oftalmolzh201816773 Eye retinal changes under the influence of chromium ions Y. V Kuzenko 1, MD post PhD; O. V. Kuzenko 2, MD; Y. A. Dyomin 2, Professor, MD, PhD 1 Medical Institute of Sumy State University; Sumy (Ukraine) 2 Kharkiv Medical Academy of Postgraduate Education; Kharkiv (Ukraine) E-mail: yevhen.kuzenko@gmail.com logvinenok_26@mail.ru TO CITE THIS ARTICLE: Kuzenko VV, Kuzenko OV, Demin YuA. Eye retinal changes under the influence of chromium ions. J.ophthalmol.(Ukraine).2018;1:67-73. https://doi.org/10.31288/oftalmolzh201816773
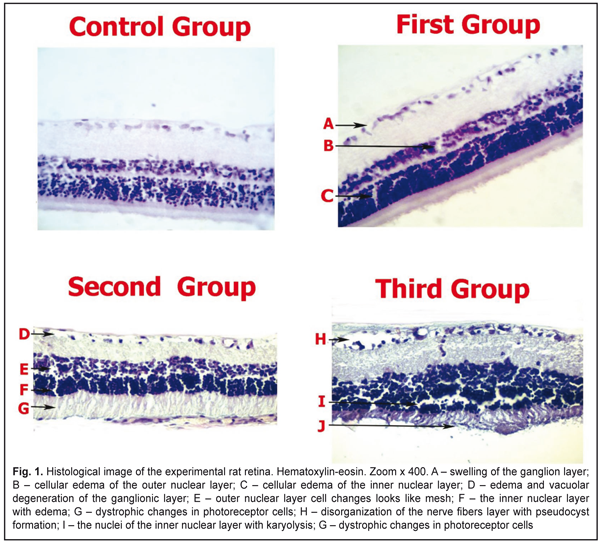
Introduction. Relationships between human eyes and metal ions take multiple forms. Neurotoxicity manifests as peripheral neuropathy, sensorineural hearing loss; ocular toxicity is presented as visual impairment. Regulation of heavy metal toxicity by the heat shock proteins (HSP90) and S100 family proteins has not been investigated in eye retina. The aim of our study was to investigate S100 and HSP90 expression in retina under the influence of chromium ion. Material and Methods. 36 (72 eyes) male albino rats that weighed 300-325 g were evaluated for histological and immunostainings for HSP90aa1 and S100. 18 rats of experimental group (36 eyes) got potassium bichromate (Sigma, USA) into drinking water in a dose of 0.02 mol/l. The rats of control group (18 individuals) drank usual drinking water. Animals were taken out of experiment at Days 20, 40 and 60 (first, second and third groups, respectively, six animals in each group) after the beginning of potassium bichromate introduction. Results. We noted the HSP90aa1 enzymatic activity in the control group. Induction of the enzymatic activity of HSP90aa1 was increased in the second group (89.7±3.5% P<0.05). S100 was expressed in the control 5.24±0.58% and experimental groups (first group - 5.67±0.32%, P˃0.05; second group - 25.72±1.54% P<0.05; third group - 34.14±2.54%, P<0.05). Conclusion. Our data conclude that HSP90aa1 and S100 proteins functionally interact during the regulation of retinal cells under the influence of chromium ion. Chromium is toxic heavy metal that has led to retinal edema. It plays a major role in retinopathy development. The potential of Chromium ion toxicity and its possible role in causing diseases of retina requires further study. Key words: eye retina, chromium ion, HSP90aa1, S100 Introduction Relationships between human eyes and metal ions take multiple forms. While a number of prior case reports and studies have explored metal ions effects on eyes, a broad comparative analysis of the relationships between metal ions and eye tissue is currently lacking. Apel W et al. have reported the clinical case of a 65-year-old male who developed retinal dysfunction following cobalt-chromium toxicity [1]. Metal ion concentrations in blood and serum have been reported with metal implants. Jantzen C et al. have reviewed the literature on blood and serum ion concentrations of chromium and cobalt following various metal hip arthroplasties [2]. The metal prostheses surfaces may be made of cobalt chromium, stainless steel, ceramic, titan or polyethylene. Corrosion can result in increased local and systemic metal concentrations. Bradberry S. M. et al have divided systemic toxicity after placement of the metal into three main categories: neuro-ocular toxicity, cardiotoxicity and thyroid toxicity. Neurotoxicity manifested as peripheral neuropathy, sensorineural hearing loss; ocular toxicity presented as visual impairment [3]. Monies A. and Prost M. have examined the influence of cobalt compound on rabbit eye tissues. The studies revealed that cobalt intoxication caused the following damages: edema and atrophy of nerve fibers, lesions of ganglion, amacrine, bipolar, horizontal cells, and nucleus of photoreceptors. The results of those experimental investigations were based only on that ophthalmological examination. [4]. Regulation of heavy metal toxicity by the heat shock proteins (HSP90) and S100 family proteins has not been investigated in eye retina. There are data published about HSP90 expression in the kidneys and liver tissues under the cobalt, molybdenum, and cadmium influence [5, 6]. Moreover, some authors believe that protein S100 displays heavy metal-induced neurotoxicity [7, 8]. The aim of our study was to investigate S100 and HSP90 expression in retina under the influence of chromium ion. Materials and Methods The study protocol was according to the provisions of "European Community Directive of 24 November 1986 on the maintenance and use of laboratory animals for research purposes". The work was implemented within the framework of research 013U003379. The subjects were 36 (72 eyes) experimental male Wistar rats that weighed 300–325 g at the onset of testing (Institute of Pharmacology, Academy of Medical Sciences, Ukraine). They were individually housed in standard cages inside a room maintained on a 12–12-hr light–dark cycle with the light part of the cycle beginning at 7 a.m. During the experiment the rats were maintained on a free water. The study protocol was approved by the Research and Ethics Committee of Sumy State University. Th rats of experimental group (18 individuals) got potassium bichromate (Sigma, USA) into drinking water in a dose of 0.02 mol/l. The rats of control group (6 individuals) drank usual drinking water. Animals were taken out of experiment at Days 20, 40, and 60 (first, second, and third groups, respectively, six animals in each group) after the beginning of potassium bichromate introduction. Hematoxylin and eosin (H&E) stains have been used for at least a century and are still essential for recognizing various tissue types and the morphologic change. Paraffin sections are prepared for H&E staining by mounting on superfrost slides, drying on a hot plate, and then immersing into three sets of xylene for 10 minutes each followed by three sets of absolute ethanol for 10 minutes and finally rinsed with tap water. The purpose is to remove the wax and dehydrate the sections. Frozen sections are stained directly without using hot plates for drying or dehydration in sets of xylene and alcohol. Slides (frozen and paraffin) are placed into haematoxylin for 5 minutes and rinsed thoroughly under tap water for approximately 4-5 minutes. Excess haematoxylin is removed by adding 1% acid alcohol (1% HCl in 70% (v/v) alcohol) for 5 seconds followed by a tap water wash. The pink haematoxylin stain is converted to blue by adding Scott’s tap water, for approximately 10 seconds until the sections turn blue. The slides are rinsed in tap water before being stained in eosin (1% (w/v)) for 15 seconds with a subsequent wash in running tap water for 1-5 minutes. The sections are then dehydrated in two washes of absolute alcohol and in two washes of xylene for 10 minutes each before being mounted in DPX mountant and covered with glass cover slips. Immunostainings for HSP90aa1 and S100 were performed on formalin-fixed (pH 7.4), paraffin-embedded thyroid tissue sections using mouse monoclonal anti- HSP90 and anti-S100 (Thermo Fisher Scientific UK). Briefly, 4μm thick tissue sections were dewaxed in xylene and were brought to water through graded alcohols. Antigen retrieval was performed by microwaving slides in 10mM citrate buffer (pH 6.2) for 30 min at high power, according to the manufacturer’s instructions. To remove the endogenous peroxidase activity, sections were then treated with freshly prepared 1.0% hydrogen peroxide in the dark for 30 min at 37° C temperature. Non-specific antibody binding was blocked using blocking serum. The sections were incubated for 30 min, at 37° C temperature, with the primaries antibodies against HSP90aa1 and S100 diluted 1:100 in phosphate buffered saline (PBS) pH 7.2, after washing 3 times with PBS. Anti-(mouse IgG)–horseradish peroxidase conjugate (1:40 000 dilution) was used for the detection of HSP90aa1 and S100 primaries antibodies, sections were then incubated for 20 min at 37° C temperature. The colour was developed by DAB. Appearance of positive factors was detected semiquantitatively by the counting of positive structures in visual field (0/- - HSP90aa1 and S100, + - few, ++ - moderate, +++ - numerous, ++++ - abundance of positive structures in visual field. Scanning Electron Microscope (SEM). Briefly, 4 μm thick tissue sections were placed on graphite plates. Paraffin sections were immersed into three sets of xylene for 10 minutes each followed by three sets of absolute ethanol for 10 minutes and finally rinsed with distilled water. Slides were placed into hematoxylin for 5 minutes and 96% alcohol for 10 minutes and rinsed thoroughly under distilled water for approximately 4–5 minutes. At the specified times, the specimens were examined for topographical changes using a SEM equipped with Energy Dispersive X-ray Spectroscope (EDS). The surface levels of copper elements were measured as weight percentage. Each sample was exposed to radiation at the center and in two additional equidistant areas at a voltage of 15KV for 60 seconds and the average figure was calculated for each specimen. The data was analysed using STATISTICA 8.0 software, user version STA862D175437Q. The results are presented as average values (±SD). The K-S test was used in order to evaluate the normality of the data. Also, the Student method was used to perform simple comparative analysis. A value of p < 0.05 was considered significant. Results Retina is shown in Fig 1. Vacuolar degeneration, disruption and pseudocyst formation occurred mainly in the layer of ganglion cells in rat's retina. Three experimental groups had pericellular swollen in outer and inner nuclear layers. The inner nuclear layer karyolysis expressed on the 60th day of the experiment. The dystrophic changes in retinal layer started at Day 20 of the experiment and intensified at Day 60.
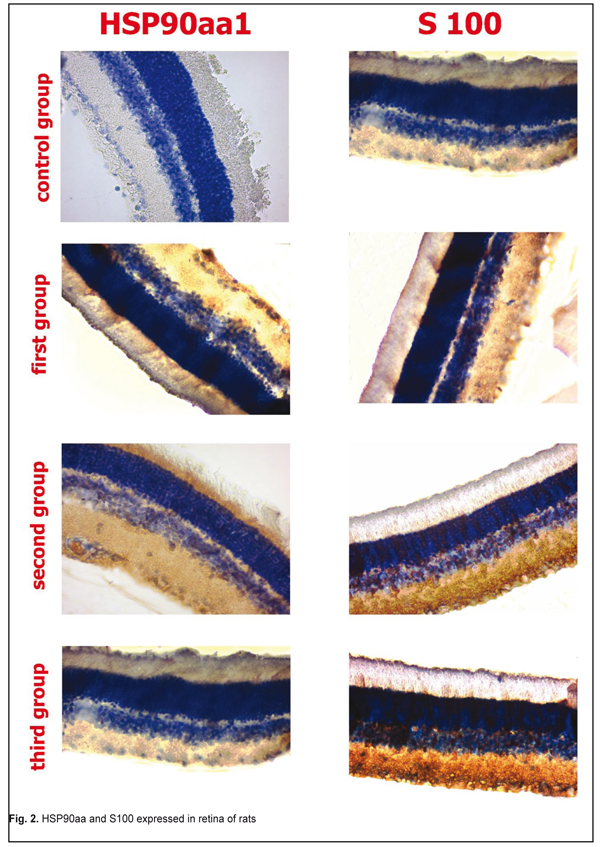
Expression of HSP90aa and S100 in retina the in first, second, and third groups are shown in Fig 2. There was no sign of malignancy or detachment in all groups.
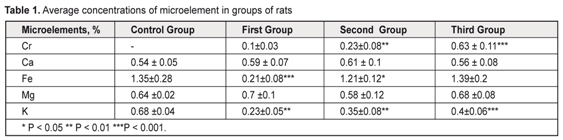
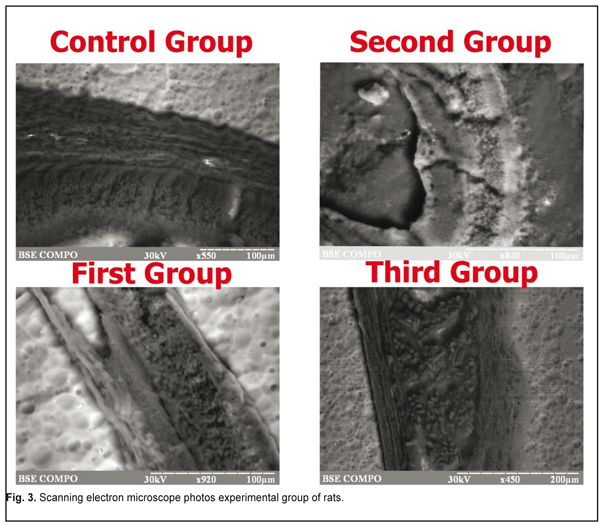
Induction of the enzymatic activity of HSP90aa1 was increased in the second group. It can be explained by the fact that HSP90aa1 is physiologically expressed by the stress factors. We didn’t find the HSP90aa1 enzymatic activity in the control group. S100 was expressed in the control (5.24±0.58%) and experimental groups (weak first group of rats). HSP90aa and S100 expressed in retina in the first group. The first group was positive for HSP90aa1 65.9±0.5% (P<0.05) in ganglion cell, inner plaxiform, and andinter nuclear layers, whereas only 5.67±0.32% of andinter nuclear layer were positive for S100 (P˃0.05). By immunohistochemistry in the second group of rat retina, 89.7±3.5% (P<0.05) of all cells layers were positive for HSP90aa1, whereas only 25.72±1.54% of andinter nuclear and outer nuclear layers cell were positive for S100 (P<0.05). The third group was positive for HSP90aa1 25.9±0.5% (P˃0.05) in ganglion cell, inner plaxiform, andinter nuclear layers, whereas only 34.14±2.54% of andinter nuclear and outer nuclear layers were positive for S100 (P<0.05). The immunoexpression of these proteins was confirmed by the presence of brown stained cytoplasm in retina cells. S100 was expressed in Mueller-cell or glial cells. In general, S100 and HSP90aa1 stainings were more intense in the second group. With respect to the immunoexpression of HSP90aa1, the rats in the third group had weak positive cells. In the rods and cones, immunoreactivity of S100 had negative reactions. S100 was expressed in the control group as retina normal function. We did not found correlation between expression S100 and HSP90aal. The average content of the micro- and macroelements under study are shown in Table 1. EDS analyses revealed that inorganic phases of retina were mainly composed of calcium and phosphorus as the major constituents with some minor components such as Cr, Ca, Fe, Mg, and K. The rat retina corresponding to Cr was higher. It can clearly be seen from Table 1 that Cr levels increased to a statistically significant extent. As for Mg and Ca levels, there was no remarkable difference between the normal and epulis tissues. The lowest levels of Fe and K were observed in the first group. SEM photos are showed on Fig 3.
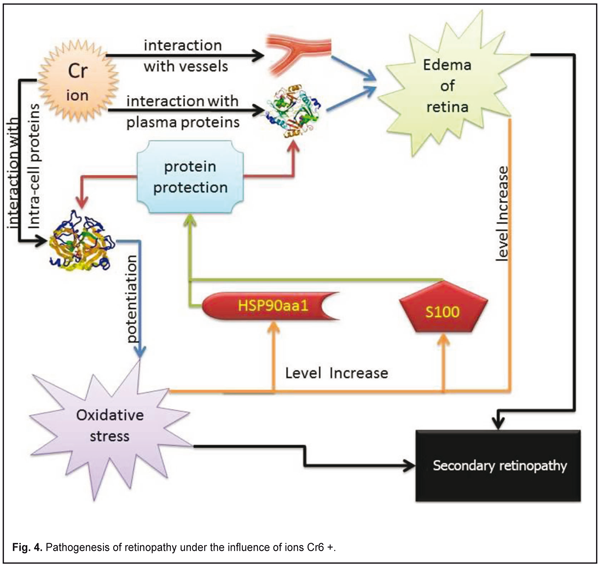
Discussion Retinopathy is associated with impaired microcirculation in the choroid and retina vessels. Vascular permeability has association with Cr ion as a result: retinal tissue transudate, subretinal edema, cell edema [9]. Retinal edema led to cell hypoxia and oxidative stress that enhanced the development of pathological changes in retina. Chromium influenced glucose, protein, and fat metabolism oxidative stress and free radical damage is thought to play a significant role in age-related macular degeneration [10]. The fluid had white or grayish area. The exudate emergence was mainly due to the release of plasma lipids through the vascular wall due to the violation of its permeability. We believe this effect is due to the binding of chromium ions from the plasma proteins. Oxidative stress was associated with chromium exposure [11]. Cr3+ and Cr6+ were considered toxic as it readily crosses cell membranes [12]. Hsp90 induced in response to various stresses [13]. In contrast to the HSPs family, Hsp90 is the most powerful ATPase activator [14]. Protein synthesis, one such vital process is often a target of oxidative stress. Generally, protein synthesis is highly regulated in adverse conditions by inhibiting production of malformed proteins and stimulating HSP90 [15]. Oxidative stress had an ambiguous effect on S100 family. Oxidative stress decreased the ability of S100 proteins to activate PP-5, which in turn modulated the ASK1-mediated signaling cascades involved in apoptosis [16]. S100A8 and S100A9 expression were upregulated in numerous cell types by oxidative stress [17]. S100A3 is highly expressed in astrocytomas. It may protect cells from oxidative damage due to very high Cys content [18]. In keratinocytes, S100A8 is induced by oxidative stress whereas S100A9 is not, confirming roles for these S100 proteins [19]. Oxidative stress induces release S100B [20]. S100B acts together with inflammatory cytokines and strengthening inflammation and causing oxidative damage to neurons [21]. As a result of our investigations and literature data, we offer the scheme of interaction between the S100 and HSP90aa1 protein (fig 4).
Conclusion Our data conclude the HSP90aa1 and S100 proteins functionally interact during the regulation of retinal cells under the influence of chromium ion. Chromium is toxic heavy metal that has led to retinal edema. It plays the main role in retinopathy development. The potential Chromium ion toxicity and possible role in diseases of the retina requires further study. References
|