J.ophthalmol.(Ukraine).2018;1:54-59.
|
https://doi.org/10.31288/oftalmolzh201815459 Visualization of ciliary body structures after preoperative anti-inflammatory in rhegmatogenous retinal detachment complicated by choroidal detachment Y. Alibet, Postgraduate Student; O.S. Zadorozhnyy, Cand Sc (Med); G.V. Levytska, Cand Sc (Med); N.V. Pasyechnikova, Dr Sc (Med), Prof., NAMS Corr Member Filatov Institute of Eye Diseases and Tissue Therapy Odessa (Ukraine) E-mail: yassinealibet@hotmail.com TO CITE THIS ARTICLE: Alibet Y, Zadorozhnyy OS, Levytska GV, Pasyechnikova NV. Visualization of ciliary body structures after preoperative anti-inflammatory treatment in rhegmatogenous retinal detachment complicated by choroidal detachment. J.ophthalmol.(Ukraine).2018;1:54-9. https://doi.org/10.31288/oftalmolzh201815459
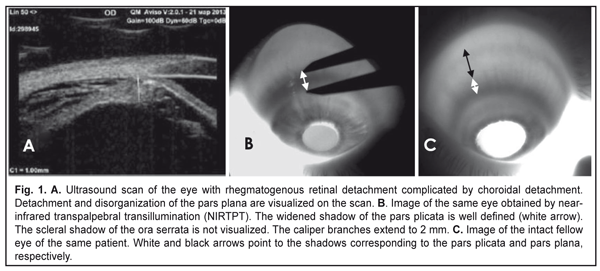
Background: Rhegmatogenous retinal detachment (RRD) can be complicated by choroidal detachment (CD). The state of the ciliary body in patients with RRD may indirectly indicate active intraocular inflammation. Purpose: To investigate ciliary body dimensions in RRD complicated by CD after preoperative anti-inflammatory treatment. Materials and Methods: This study included 31 RRD+CD patients (31 eyes) with an intact fellow eye who were under observation. Before the vitrectomy, they received 4-mg intravitreal triamcinolone (IVT), either alone or in combination with 0.5 to 0.8 mL of perfluorpropane. Near-infrared transpalpebral transillumination (NIR TPT) and ultrasound biomicroscopy (UBM) of anterior eye structures were performed before and 1, 2, 3 or 4 days after preoperative intravitreal therapy. Results: Mean baseline width and mean baseline thickness of the pars plicata in eyes with combined RRD and CD were statistically significantly greater than in intact fellow eyes (2.4 ± 0.1 mm versus 1.9 ± 0.1 mm, P = 0.01, and 0.83 ± 0.1 mm versus 0.65 ± 0.1 mm, P = 0.02, respectively). Mean post-treatment pars plana width in the affected eyes having axial length > 25.0 mm was 4.74 ± 0.63 mm, and the percentage of these eyes was 64.5% among eyes with combined RRD and CD. Mean thickness and mean width of the pars plicata in the affected eyes after treatment were 2.2 ± 0.1 mm и 0.66 ± 0.1 mm, respectively. Conclusions: In eyes with combined RRD and CD, pars plicata width and thickness were greater than in intact fellow eyes. Ciliary body detachment tends to occur in longer eyes (therefore, those with a greater pars plana width) with RRD. In eyes with combined RRD and CD, there was evidence of re-attachment of the pars plana as well as reduction in pars plicata thickness and width after preoperative anti-inflammatory treatment. Keywords: rhegmatogenous retinal detachment, ciliary body, infrared radiation, transillumination Introduction Rhegmatogenous retinal detachment (RRD) can be complicated by choroidal detachment (CD) and accompanied by hypotony and intraocular inflammation. This is the most unfavorable type of RRD for prognosis and requires a special treatment strategy. Most probably, a blood ocular barrier breakdown is an early event of CD in the presence of RRD, which explains the efficacy of preoperative anti-inflammatory therapy [1]. Therefore, the state of the ciliary body in patients with RRD may indirectly indicate active intraocular inflammation. The dimensions of ciliary body structures may be objectively assessed by ultrasound scanning or diaphanoscopy (e.g., near-infrared transpalpebral transillumination) [2, 3]. Ultrasound biomicroscopy (UBM) enables precise imaging of anterior eye structures and quantitative measurements. In ultrasound examination, ciliary processes are visualized, and their location, size and shape are identified. In addition, it enables assessing the thickness of cliliary body structures [3]. In RRD complicated by CD, UBM revealed that ciliary body detachment was characterized by the detachment of the pars plana only, whereas attachment of the pars plicata to the sclera was maintained [1]. Diaphanoscopy enables imaging the projection of the ciliary body onto the sclera and assessing the widths of ciliary body structures. Transcorneal or transscleral illumination should be used for visible globe transillumination. Near-infrared transpalpebral transillumination (NIR TPT) enables non-invasive ciliary body imaging and precise assessment of scleral projections of ciliary body structures [3]. The purpose of this study was to investigate ciliary body dimensions in RRD complicated by CD after preoperative anti-inflammatory treatment. Materials and Methods The study was approved by a local Bioethics Committee of the Filatov Institute and included 31 RRD+CD patients (31 eyes) with an intact fellow eye who were under observation. Visual acuity measurement, biomicroscopy, ophthalmoscopy, IOP measurements, NIR TPT and UBM of the ciliary body, choroid and retina were performed for both eyes in all patients before treatment. The ciliary body was visualized with 940-nm NIR TPT [3]. The NIR TPT system used consisted of (1) a wireless LED IR light source with a dominant wavelength of 940 nm, (2) slit-lamp attachable monochrome video camera capable of recording near-IR images and video, and (3) computer with the software for processing the video captured from the camera and transferring the processed signal to the display [4]. Images of scleral shadows of the pars plicata and pars plana extending to the ora serrata were taken and saved in the computer. The widths of the shadows of ciliary body structures were measured with calipers. These measurements were made at four meridians: 12 o’clock, 6 o’clock, nasally (3 o'clock OD and 9 o'clock OS), and temporally (9 o'clock OD and 3 o'clock OS), with the patient sitting at a slit lamp [5]. Ciliary body thickness measurements were made between the ciliary processes located most closely to the scleral spur at four meridians (12 o’clock, 6 o’clock, nasally (3 o'clock OD and 9 o'clock OS), and temporally (9 o'clock OD and 3 o'clock OS)). We used the Aviso UBM unit (Quantel Medical) with a 50-MHz linear probe (axial resolution: 35 µm; lateral resolution: 60 µm). On examination, the patient lying on his back, with the head of the bed raised [1]. Epibulbar anesthesia with ophthalmic 0.5% proparacaine hydrochloride was used in both eyes before UBM examination. Before vitrectomy, patients received 4-mg intravitreal triamcinolone (IVT), either alone or in combination with 0.5 to 0.8 mL of perfluorpropane until IOP became normotensive. The intravitreal anti-inflammatory treatment used in this study aimed to remove intraocular inflammation and resolve the choroidal detachment in order to reduce the risk of intra- and post-operative complications of retinal detachment surgery [1]. One, 2, 3, or 4 days after intravitreal treatment, patients underwent repeat NIR TPT and UBM of the ciliary body, choroid and retina. In addition, the axial length of the eye was measured with ultrasonic biometry. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. The level of significance p ≤ 0.05 was assumed. Data are presented as mean ± standard deviation (SD). Results Ultrasound examination revealed (a) detachment and disorganization of the pars plana and (b) maintained attachment of the pars plicata to the sclera before preoperative anti-inflammatory pretreatment. Pars plana detachment in two or more quadrants was found in all affected eyes. NIR TPT failed to determine the margin of the pars plana in these quadrants because the scleral shadow of the ora serrata was not visualized, but the width of the pars plicata was determined in all quadrants.
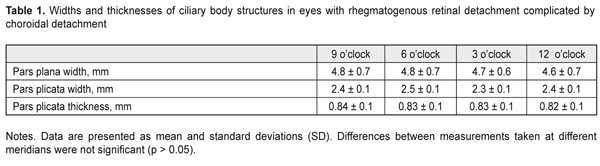
Mean baseline width of the pars plicata in eyes with both RRD and CD was statistically significantly greater than in intact fellow eyes (2.4 ± 0.1 mm versus 1.9 ± 0.1 mm, P = 0.01). We managed to determine the baseline width of the pars plana at the site of the attachment of the ciliary body to the sclera in 29 eyes with both RRD and CD, with the mean value being almost the same as that in intact fellow eyes (4.7 ± 0.7 mm versus 4.65 ± 0.3 mm, P = 0.6). Mean baseline thickness of the pars plicata in eyes with both RRD and CD was statistically significantly higher than that in intact fellow eyes (0.83 ± 0.1 mm versus 0.65 ± 0.1 mm, P = 0.02). NIR TPT measurements of the widths and thicknesses of ciliary body structures at different meridians in eyes with RRD are presented in Table 1.
All affected eyes were hypotonic at baseline. One, 2, 3 or 4 days following intravitreal injections, mean IOP in affected eyes improved from 7.7±1.7 mmHg at baseline to 14 ± 1.0 mmHg (P = 0.0001), whereas that in intact fellow eyes did not change (18 ± 1.0 mmHg). In all affected eyes, UBM revealed improvement in CD after intravitreal therapy, although the retinal detachments were still present. In every case, the pars plana was found to be re-attached to the sclera at all the four meridians. In addition, mean ciliary body thickness in the affected eyes decreased from 0.83 ± 0.1 mm at baseline to 0.66 ± 0.1 mm at day 1, 2, 3 or 4 after anti-inflammatory treatment (P < 0.001). The axial length of the eye was ultrasonically measured after post-treatment restoration of IOP to normal levels. Mean axial length of the affected eye was 26.9 ± 3.3 mm, with 11 eyes (35.5%) and 20 eyes (64.5%) having axial length < 24.9 mm and > 25 mm, respectively. Mean axial length of intact fellow eyes was 27.0 ± 3.0 mm. NIR TPT managed to visualize the ora serrata shadow in all the quadrants after post-treatment re-attachment of the pars plana, which allowed us to better define the margins of ciliary body structures. In eyes with RRD, mean pars plicata width increased after preoperative anti-inflammatory treatment to 2.2±0.1 mm (P < 0.05), whereas mean pars plana width did not change compared to baseline (4.68 ± 0.7 mm, P > 0.05). Mean post-treatment pars plana width in the affected eyes having axial length < 24.9 mm was lower than in those having axial length > 25.0 mm (4.2±0.6 mm versus 4.74 ± 0.63 mm, P = 0.033). Mean pars plana width in the intact fellow eyes was 4.65 ±0.3 mm and was not significantly different from that in the affected eyes (P > 0.05). Discussion The choroidal and ciliary body morphometrics are known to depend on axial length [5-9]. OCT-based measure of macular choroidal thickness has been found to decrease in eyes with increased axial length of the eye [9]. In addition, a relationship has been reported between ciliary body thickness and axial length. Thus, Oliveira and co-authors [10] demonstrated that ciliary body thickness increases with increasing axial myopia. Ernst and colleagues [11] have found that the relationship between ciliary body thickness and refractive error was of a non-linear nature: the ciliary body was thicker in patients with low to moderate myopia; in higher levels of myopia and the largest axial lengths, however, ciliary body thickness was similar to that found in emmetropia. It has been reported that, in anisometropia, there was no significant difference in ciliary body thickness between fellow eyes [12]. A direct relationship exists between pars plana width and axial length. Thus, previously, using NIR TPT, we have found that, in healthy persons with axial length of 20-22.9 mm, 23-24.9 mm, and above 25 mm, (1) the pars plana width was 3.1 mm, 4.1 mm, and 5.0 mm, respectively, and (2) pars plicata width did not depend on the axial length [5]. In addition, (1) a direct relationship was found between pars plana width and axial length in eyes with RRD, and (2) there was no difference in pars plana width or pars plicata width between the eye with RRD and the intact fellow eye [5]. Furthermore, in eyes with RRD, the pars plicata thickness was greater than in intact fellow eyes [13]. In our current study, the NIR TPT-based mean pars plicata width and the UBM-based ciliary body thickness in eyes with RRD complicated by CD were significantly greater than those in intact fellow eyes (2.4 ± 0.1 mm versus 1.9 ± 0.1 mm, P = 0.01, and 0.83 ± 0.1 mm versus 0.65 ± 0.1 mm, P = 0.02, respectively). Therefore, pars plicata width and thickness in eyes with RRD complicated by CD were significantly greater than those in intact fellow eyes. Given also the ultrasonic evidence of ciliary body changes like detachment and disorganization of the pars plana in all study in eyes with RRD complicated by CD, one should suspect the presence of intraocular inflammation-related ciliary body edema with blood ocular barrier breakdown. The evidence of structural changes in the ciliary body in eyes with this disorder confirms the appropriateness of the preoperative anti-inflammatory treatment that has been previously recommended [1]. In the present study, (1) mean IOP in affected eyes improved from 7.7±1.7 mmHg to 14 ± 1.0 mmHg (P = 0.0001) and (2) there was a UBM evidence of re-attachment of the ciliary body and reduction in ciliary body edema after preoperative anti-inflammatory treatment. In addition, there were improvements in pars plicata width and thickness, which demonstrates the efficacy of the therapy. It is noteworthy that the percentage of eyes longer than 25 mm (i.e., eyes with a wider pars plana) was 64.5% among eyes with RRD complicated by CD. Thus, mean post-treatment pars plana width in the affected eyes having axial length > 25.0 mm was 4.74 ± 0.63 mm. Therefore, one may hypothesize that the relative risk of ciliary body detachment rises with increasing axial length, and, accordingly, with increasing pars plana width in eyes with RRD with a breakdown of the blood ocular barrier. Conclusions First, in eyes with RRD complicated by CD, (1) pars plana detachments were observed, and (2) pars plicata width and thickness were greater than in intact fellow eyes, which may be evidence of intraocular inflammation. Second, ciliary body detachment tends to occur in longer eyes (therefore, those with a greater pars plana width) with RRD complicated by CD. Thus, the percentage of eyes longer than 25 mm (i.e., eyes with a wider pars plana) was 64.5% among eyes with RRD complicated by CD.
Finally, in eyes with RRD complicated by CD, there was evidence of re-attachment of the pars plana as well as reduction in pars plicata thickness and width after preoperative anti-inflammatory treatment.
References
|