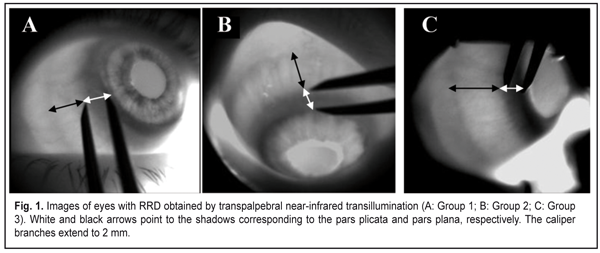
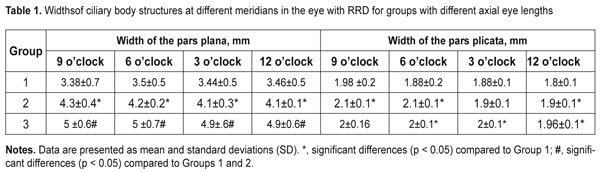
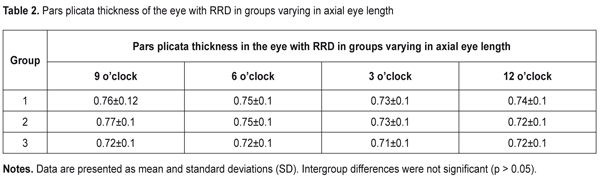
J.ophthalmol.(Ukraine).2017;6:32-36.
|
https://doi.org/10.31288/oftalmolzh201763236 Dimensions of ciliary body structures in various axial lengths in patients with rhegmatogenous retinal detachment O.S. Zadorozhnyy, CandSc (Med), Alibet Yassine, Postgraduate Student, A.S. Kryvoruchko, G.V. Levytska, CandSc (Med), N.V. Pasyechnikova, DrSc (Med), Prof. Filatov Institute of Eye Disease and Tissue Therapy Odesa, Ukraine E-mail: laserfilatova@gmail.com Background: Ciliary body size may be objectively assessed by ultrasound scanning and near-infrared transpalpebral transillumination. Purpose: To investigate the dimensions of ciliary body structures in various axial eye lengths in patients with rhegmatogenous retinal detachment (RRD). Materials and Methods: This study included 35 RRD patients (35 eyes) with an intact fellow eye who were under observation. These were divided into three groups, based on axial length of the eye with RRD. All patients underwent near-infrared transpalpebral transillumination (NIR TPT) and ultrasound scanning of the anterior eye. Results: In patients with axial length ranging from 20 mm to 22.9 mm, 23 mm to 24.9 mm, and larger than 25 mm, pars plana width was 3.4 mm, 4.16 mm, and 4.95 mm, respectively, whereas pars plicata width was 1.9 mm, 1.99 mm, and 2.0 mm, respectively, and pars plicata thickness was 0.75 mm, 0.75 mm, and 0.72 mm, respectively. Conclusions: A direct relationship exists between pars plana width and axial length, but pars plicata thickness and width do not depend on the axial length in eyes with RRD. There was no difference in pars plana width or pars plicata width between the eye with RRD and unaffected fellow eye. In the eye with RRD, the pars plicata thickness was thicker than that in the intact fellow eye, which may evidence the presence of intraocular inflammation. Keywords: rhegmatogenous retinal detachment, infrared radiation, near-infrared transpalpebraltransillumination Introduction Rhegmatogenous retinal detachment (RRD) is known sometimes to be complicated by ciliochoroidal detachment in the presence of intraocular inflammation, with ultrasonographic evidence of the pars plana detachment and disorganization and increased thickness of the pars plicata. Most probably, a blood ocular barrier breakdown is an early event of ciliochoroidal detachment in the presence of RRD, which explains the efficacy of preoperative anti-inflammatory treatment [1]. Therefore, the state of the ciliary body in patients with RRD may indirectly indicate an active intraocular inflammatory process. Ciliary body size may be objectively assessed by ultrasound scanning or near-infrared transpalpebral transillumination [2, 3]. Ultrasound biomicroscopy enables precise imaging of anterior eye structures and quantitative measurements. In ultrasound examination, ciliary processes are visualized, and their location, size and shape are identified. In addition, it enables assessing the thickness of cliliary body structures [2]. Diaphanoscopy (near-infrared transpalpebral transillumination) enables imaging the projection of the ciliary body onto the sclera and assessing the widths of ciliary body structures. Transcorneal or transscleral illumination should be used for visible globe transillumination. Near-infrared transpalpebral transillumination (NIR TPT) enables non-invasive ciliary body imaging and precise assessment of scleral projections of ciliary body structures [3].
The purpose of this study was to investigate the dimensions of ciliary body structures in various axial eye lengths in patients with rhegmatogenous retinal detachment. Materials and Methods The study was approved by a local Bioethics Committee of the Filatov Institute and included 35 RRD patients (35 eyes) with an intact fellow eye who were under observation. Patients with anisometropia were excluded from the study. The patients were divided into three groups, based on axial length (AL) of the eye with RRD: 10 patients with AL ranging from 20 mm to 22.9 mm (Group 1), 10 patients with AL ranging from 23 mm to 24.9 mm (Group 2), and 10 patients with AL larger than 25 mm. All patients underwent ultrasound scanning and NIR TPT of both eyes. The ciliary body was visualized with 940-nm NIR TPT [3]. The NIR TPT system used consisted of (1) a wireless light-emitting-diode infared light source with a dominant wavelength of 940 nm,(2) slit-lamp attachable monochrome video camera capable of recording NIR images and video, and (3) computer with the software for processing the video captured from the camera and transferring the processed signal to the display [4]. Images of scleral shadows of the corona ciliaris and pars plana extending to the ora serrata were taken and saved in the computer. The widths of the shadows of ciliary body structures were measured with calipers. These measurements were made at four meridians: 12 o’clock, 6 o’clock, nasally (3 o'clock OD and 9 o'clock OS), and temporally (9 o'clock OD and 3 o'clock OS), with the patient sitting at a slit lamp [5]. Ciliary body thickness measurements were made between the ciliary processes located most closely to the scleral spur at four meridians (12 o’clock, 6 o’clock, nasally (3 o'clock OD and 9 o'clock OS), and temporally (9 o'clock OD and 3 o'clock OS)), with the patient lying on his back, and with the head of the bed raised [1]. We used the Aviso UBM unit (Quantel Medical) with a 50-MHz linear probe (axial resolution: 35 µm; lateral resolution: 60 µm).Epibulbar anesthesia with ophthalmic 0.5% proparacaine hydrochloride was used in both eyes. Statistical analysis was performed using STATISTICA 10 software (StatSoft, Inc.). Group means and standard deviations (SD) were calculated for all variables. The level of significance p ? 0.05 was assumed. Results The mean axial length in the eye with RRD was significantly longer in Group 2 compared to Group 1 (23.8 ± 0.4 mm vs 22.3 ± 0.5 mm; p < 0.0001), and in Group 3 compared to Group 2 (28.5 ± 3.2 mm vs 23.8 ± 0.4 mm, p = 0.0001). The mean axial length in the fellow eye was significantly longer in Group 2 compared to Group 1 (23.8 ± 0.5 mm vs 22.2 ± 0.5 mm; p < 0.0001), and in Group 3 compared to Group 2 (28.7 ± 3.0 mm vs 23.8±0.5 mm, p = 0.0001). The width of the pars plana in the eye with RRD was significantly wider in Group 2 compared to Group 1 (4.16 ± 0.6 mm vs 3.4 ± 0.4 mm; p < 0.0001), and in Group 3 (4.95 ± 0.6 mm) compared to Group 1 (p < 0.0001) and Group 2 (p < 0.0001). The width of the pars plana in the fellow eye in Group 1, Group2 and Group 3 was 3.5 ± 0.7 mm, 4.2 ± 0.3 mm, and 4.85 ± 0.8 mm, respectively, and was not significantly different from that in the eye with RRD (p = 0.87; p = 0.82,and p =0.7, respectively). There was no significant difference in the width of the pars plicata between the eye with RRD and the fellow eye in Group 1 (1.9 ± 0.2 mm vs 1.9 ± 0.2 mm, p = 0.9), Group 2 (1.99 ± 0.1 mm vs 2.0 ± 0.1 mm, p = 0.8), and Group 3 (2.0 ± 0.1 mm vs 2.1 ± 0.1 mm, p = 0.2). NIR TPT measurements of the widths of ciliary body structures at different meridians in eyes with RRD in the three groups are presented in Table 1. The mean thickness of the pars plicata in the eye with RRD in Group 1 (0.75 ± 0.1 mm) was the same as that in Group 2 and thicker than that in Group 3 (0.72 ± 0.1 mm; p = 0.9). The mean thickness of the pars plicata in the eye with RRD was significantly (p < 0.05) thicker than that in the fellow eye in the three groups. Ultrasound measurements of the thicknesses of the pars plicata at different meridians in eyes with RRD in the three groups are presented in Table 2.
Discussion Morphometric characteristics of the choroid are known to depend on the size of the eye. Thus, OCT-measured subfoveal choroidal thickness has been found to decrease with an increase in axial length [6]. A relationship between pars plana width and axial length has been also reported [5, 7-8]. The pars plana width (P<0.01) was significantly shorter in hyperopes (defined as: 21.5-22.9 mm axial length) than in high myopes (>24.8 mm axial length) in a prospective case series on 450 enucleated adult eyes [9]. In addition, there was a strong positive correlation between the axial length and the pars plana width, both temporally and nasally (P<0.01) [9]. Previously, we have reported that the NIR TPT-measured mean pars plana width in healthy individuals with the axial length of 20-22.9 mm, 23-24.9 mm, and above 25 mm was 3.1 mm, 4.1 mm, and 5 mm, respectively [5]. However, no relationship between the pars plicata thickness or width and axial length has been found [5]. In the current study, the width of the pars plana and the width of the pars plicata in the fellow eye in the three groups were not significantly different from those in the eye with RRD (p > 0.05). A relationship between ciliary body thickness and axial length has been reported in the literature. Oliveira et al [10] found ciliary body thickness to increase with increasing axial myopia and axial length. It has been reported that, in anisometropia, an eye can grow longer and more myopic than its fellow eye without resulting in an increase in ciliary muscle thickness [11]. Ernst and colleagues [12] have found that the relationship between ciliary body thickness and refractive error was of a non-linear nature: the ciliary body was thicker in patients with low to moderate myopia; in higher levels of myopia and the largest axial lengths, however, ciliary body thickness was similar to that found inemmetropia. In the current study, there was no significant difference in the thickness of the pars plicata among the groups, and the thickness of the pars plicata in the eye with RRD was significantly (p < 0.05) thicker than that in the fellow eye in the three groups. Previously, it has been reported [1] that, in RRD combined with ciliochoroidal detachment, hypotony was found, and ultrasound examination revealed disorganization of the pars plana and increase in ultrasound-measured thickness of the pars plicata to as much as 0.83 mm before preoperative anti-inflammatory pretreatment. In addition, preoperative anti-inflammatory pretreatment resulted in re-attachment of the ciliary body, with a reduction in the ciliary body edema to 0.65 mm [1]. In the current study, the ciliary body thickness in the eye with RRD was 0.72-0.75 mm. Therefore, in the eye with RRD (and without ciliochoroidal detachment), the ciliary body thickness was thicker than that in the intact fellow eye, and than that in eyes of healthy individuals, which may evidence the presence of ciliary body edema in the presence of intraocular inflammation and blood ocular barrier breakdown. Conclusions First, a direct relationship exists between pars plana width and axial length in eyes with RRD. Thus, in eyes with RRD and axial length of 20-22.9 mm, 23-24.9 mm, and above 25 mm, (1) the ciliary body thickness was 3.41 mm, 4.16 mm, and 4.95 mm, respectively, and (2) pars plicata thickness and width did not depend on the axial length. Second, there was no difference in pars plana width or pars plicata width between the eye with RRD and unaffected fellow eye. Finally, in the eye with RRD, the pars plicata thickness was thicker than that in the intact fellow eye, which may evidence the presence of intraocular inflammation.
References
|