J.ophthalmol.(Ukraine).2017;6:25-31.
|
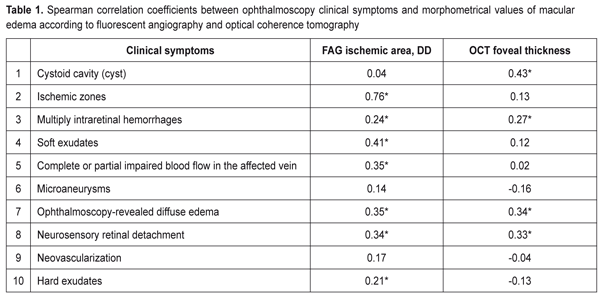
https://doi.org/10.31288/oftalmolzh201762531 Criteria of severity for macular edema associated with retinal vein occlusion: cohort heterogeneity and efficacy evaluation of threshold and subthreshold retinal laser photocoagulation depending on the macular edema severity criteria. Part II. T.O. Romanova, a Post Graduate student SI “Filatov Institute of Eye Diseases and Tissue Therapy” Odessa, Ukraine E-mail: romanova-work@rambler.ru Background. Macular edema (ME) develops in 60-100% of patients with retinal vein occlusion (RVO). ME becomes chronic with a decrease in visual acuity in 1/3-2/3 of patients with RVO-associated ME. The gold standard of care to treat patients with macular edema associated with RVO is retinal laser photocoagula-tion. Purpose. To develop an evaluation system for severity of macular edema asso-ciated with retinal vein occlusion on a ground of morphometrical and clinical characteristics and to compare the efficacy of threshold and subthreshold retinal laser photocoagulation depending on this evaluation. Material and Methods. A total of 160 patients (160 eyes) with RVO-associated macular edema were included in the study. Laser photocoagulation was performed not before than three months of disease onset. Subthreshold and threshold laser photocoagulation of the macula was performed in 38 and 110 patients, respectively. 12 patients had resorption of edema so they required no laser treatment. The follow-up period was twelve months. The examination methods used have been described in our previous paper. Results. The heterogeneity analysis of the cohort of patients with RVO-associated ME was proposed. The clinical burden assessment was developed and criteria of macular edema severity were defined. There were four clusters that clearly differed in their clinical and morphometric characteristics. Subthreshold laser photocoagulation was shown to be more successful for patients with significant retinal ischemia and threshold laser photocoagulation was more successful for patients with the increased foveal thickness. Conclusions. Four cluster distribution of the RVO-associated ME patients made it possible to reduce the heterogeneity of the studied cohort since the defined clusters were homogenous in severity criteria; the patients had similar values within each cluster. Subthreshold photocoagulation was best for the significant ischemic area (>5 DD). For the foveal thickness >547 µm, most successful was threshold laser photocoagulation. Keywords: macular edema, retinal vein occlusion, laser retinal photocoagulation, clinical symptoms, severity criteria Background Macular edema is a common consequence of retinal vein occlusion (RVO) which in most cases leads to vision loss [10, 13]. According to literature data, macular edema develops in 60-100% of RVO cases; moreover, it survives for a year and becomes chronic in 1/3-2/3 of patients, being a leading cause of patient’s impaired vision [8]. The gold standard of care to treat patients with macular edema (ME) associated with RVO is retinal laser photocoagulation [9]. When choosing a treatment tactic for RVO-associated macular edema, ophthalmologists consider an area of edema (focal or diffuse) and patient’s visual acuity; another indication for retinal laser photocoagulation in diabetic maculopathy is a presence of clinically significant macular edema in the retina [7]. A standard classification takes into account only an area of retinal ischemia, estimated by fluorescent angiography (FAG), and does not reflex, in a full degree, a degree of edema severity. Clinical symptoms in the macular and optical coherence tomography (OCT) retinal thickness which could help in a selection of laser treatment and individual approach to each patient are not considered. Purpose. To develop an evaluation system for severity of macular edema associated with retinal vein occlusion on the ground of morphometrical and clinical characteristics and to compare the efficacy of threshold and subthreshold retinal laser photocoagulation depending on this evaluation. Material and Methods A total of 160 patients (160 eyes) with RVO-associated macular edema, having unsuccessfully undergone a conservative treatment course, were included in the study. Laser photocoagulation was performed not before than three months of disease onset, so the study excluded patients with macular edema against acute RVO. 12 patients had resorption of edema while preparing to laser photocoagulation so they required no laser treatment. Subthreshold and threshold laser photocoagulation of the macula was performed in 38 and 110 patients, respectively. The follow-up period was twelve months. The examination methods used have been described in our previous paper [5]. To perform statistical analysis we used: Spearman rank correlation analysis, contingency table analysis with Pearson's chi-square test, hierarchical cluster analysis, and k-means cluster analysis. Dispersion analysis followed by the Newman-Keuls multiple comparison criterion (the mean values are presented with a mean-square error ± m) was used to analyze the differences in the quantitative parameters in the 4 groups. To assess the treatment success by post treatment visual acuity changes we used octave calculations registering visual acuity before and after treatment [2, 4]. Octaves were compared using the Mann-Whitney rank-sum test (medians are given with quartiles in brackets). To create ME severity classification rules, the optimal values for the distribution of quantitative characteristics were obtained using ROC analysis with the calculation of sensitivity and specificity for optimal values. Statistical analysis was performed using Statistica 10.0 and MedCulc 9.0 software programs. Results and Discussion As it has been shown in our previous paper [5], based on the baseline examination, a cohort of the studied patients was characterized by a great variety of retinal pathological changes, which would require a differentiating approach to laser treatment for these patients. The standard classification of RVO-associated macular edema is based on determining the area of edema and the area of retinal ischemia in the macula [12]. The retinal ischemia area is critical for predicting neovascularization development; however, it is not clear how a type of RVO affects ME development and laser treatment success. That is why a search for new factors related both to assessment of disease severity and to prediction of treatment success is required. RVO-associated macular edema is accompanied by many clinical symptoms, and the same symptom can be expressed in various degrees, moreover, this pathological picture is difficult for reading and analysis and a great variety of individual symptom complexes complicates efforts to systematize them. For the same reason, it is difficult to assess the effect of certain pathological systems on visual acuity of a patient. Difficulties in interpreting these connections have led to a widespread thought of a low value of clinical symptoms in the retina for determining a treatment tactic [11]. At the first stage of the study, we analyzed a relation between clinical symptoms and FAG and OCT data. Clinical parameters included in the study are binary features (1 – the presence of a feature; 0 – no feature). Table 1 demonstrates coefficients of rank correlation between clinical and morphological signs. As it can be seen from the Table 1 data, a great part of clinical symptoms have statistically significant correlation coefficients with quantitative assessment of the pathology studied so they can be used in developing the evaluation system for macular edema severity. So, as follows from the data presented in Table 1, the presence of cysts correlated with the OCT fovea thickness (r = 0.43) and did not with ischemic area (r = 0.04). Correlation relationship analysis revealed that the studied clinical symptoms were, in different degree, associated with both the ischemic area and the OCT macular edema size. So, to assess the macular edema severity on grounds of ten clinical symptoms, we used a weighted by some features sum (clinical symptoms 1, 2, 3, and 7 in Table 1) of all clinical symptoms to obtain an integral quantitative assessment of RVO-associated macular edema severity. As a result, we received a characteristic which had a value from 1 to 13 units in the cohort.
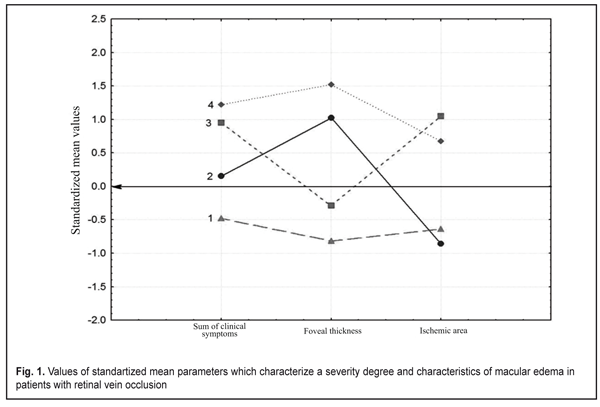
At the next study stage we used an automatic grouping method according to simultaneously three features characterizing both a clinical picture (a sum of clinical symptoms) and morphometrical characteristics of the OCT edema thickness and the FAG ischemic area. These study involved only those patients who had FAG and OCT examinations (n=61). The automatic grouping method based on the modern computer technology makes it possible to simultaneously analyze mass data (feature complex) characterizing a pathologic process. Such an approach to classification in ophthalmology has been realized by Vit V.V. et al. when clustering patients with uveal melanoma [1] and by Pasyechnikova N.V. et al when developing a classification of blood-retinal barrier disorders in diabetic retinopathy patients [3]. At the first stage of automatic grouping we used hierarchical cluster analysis (Ward’s method, Manhattan distance) which demonstrated that the studied cohort could be divided into two big clusters or into four clusters with a smaller intercluster distance. At the next stage we used k-means analysis with a set number of clusters equaling 4 since four clusters could reflex the character of RVO-associated ME better than two clusters. Figure 1 demonstrates that the patients included in Cluster 1 were characterized by the lowest values of all parameters (all mean values are below a zero line), which evidences of the mildest disease course. At the same time, it should be noted that the patients included in Cluster 4 had all values above the zero line, which indicates the most severe disease course with both great clinical burden and the presence of significant edema and ischemic zones. The patients of Clusters 2 had significant foveal edema with a small area of ischemia while the patients of Cluster 3 had a significant ischemic area with a smaller size of foveal edema (Fig. 1) Figure 1 also shows that the FAG ischemic zone was similar for the patients of Cluster 1 and 2 and was characterized by the small ischemic area while the patients of both Clusters 3 and 4 are characterized by the significant ischemic area. The foveal thickness was similar for the patients of Cluster 2 and 4 (above the line in cohort) while the patients of Cluster 1 and 3 were characterized by mild foveal edema. It should also be noted that the developed integral evaluation of clinical symptoms (a sum of clinical symptoms) displayed the disease severity (Fig.1). So, the minimal clinical symptoms sum in Cluster 1 patients was combined with minimal FAG and OCT disorders and the maximal clinical symptoms sum in Cluster 4 patients was combined with maximal FAG and OCT disorders. It can be concluded that this evaluation is largely associated with the ischemic area and in a less degree with the edema thickness.
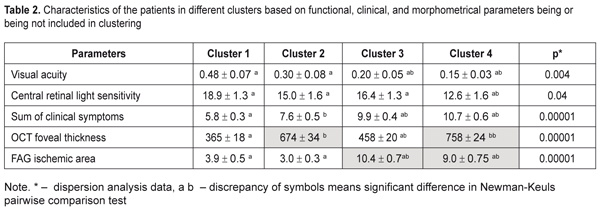
An automatic grouping algorithm for the RVO-associated ME patients set four clusters which were a mathematical classification of these patients according to three features at once. Table 2 demonstrates the mean values for the features and a difference value in each cluster. Statistical significant differences of the mean values were determined not only according to features included in clustering but also to functional characteristics. So, Cluster 1 patients had the highest values of visual acuity (VA) and retinal light sensitivity (RLS), which corresponded to minimal clinical burden according to ophthalmoscopy, as well as the smallest foveal thickness and ischemic area. Data given in Table 2 demonstrate that the VA and RLS functions decreased from Cluster 1 to Cluster 4, herewith, the sum of clinical symptoms increased from 5.8 to 10.7 scores. At the same time, OCT foveal edema statistically significantly differed in patients of the four clusters. Also, Cluster 2 patients had more pronounced edema than Cluster 3 patients. Cluster 4 patients had the most significant changes as it can be seen in Table 2 and in Figure 1.
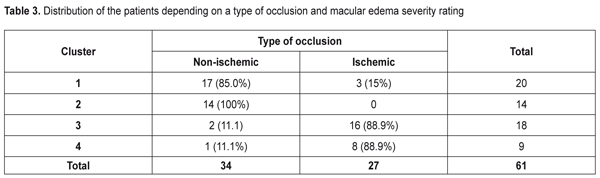
As to the area of the ischemic zone, it was significantly smaller in the patients in Clusters 1 and 2 than in Cluster 3 and 4 patients. These significant changes in the ischemic area between the cluster pairs can be explained by the fact that in the studied cohort there were patients with both ischemic and non-ischemic edema. Contingency between the standard macular edema classification according to the occlusion type and the automatic grouping clusters is given in Table 3.
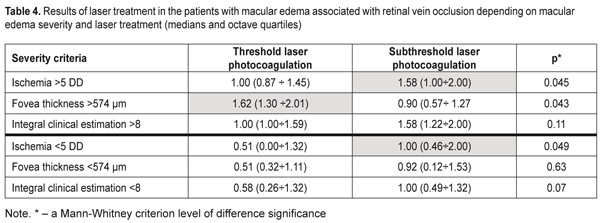
Data in Table 3 demonstrate that the clusters of the patients had high-level statistically significant contingency with the standard RVO classification of the macular edema (?2= 32.9; р = 0.000001). The patients in Clusters 1 and 2 corresponded to the non-ischemic RVO type in 85% and 100 % of cases, respectively, with the ischemic area of 3.9 and 3.0 disc diameters (DD), respectively. Cluster 3 and 4 patients had the ischemic RVO type in 88.9 % of cases with the mean ischemic area of 9.0 and 10.4 DD, respectively (Table 2). The clusters contain information about an RVO type, clinical burden and visual function disorders and they also give the possibility to assess edema severity based on the foveal thickness. Thus, as it can be seen in Table 2, in the two first clusters, which are non-ischemic, the foveal thickness differed significantly. In other words, having the similarly small ischemic area, there was a significant difference in the foveal thickness of more than 300 µm. These differences in the edema severity essentially completed the standard classification which had not considered the OCT data. A pair of Clusters 3 and 4 were different in the foveal thickness and they both were characterized by great ischemia; however, each of them corresponded to a different degree of the OCT foveal edema. Thus, analysis data on a character of macular edema in the RVO patients make it possible to conclude that the clusters not only are different in the character of pathological changes but these changes can influence the treatment success. To check this assumption on the ground of foveal thickness and ischemic area values, the cohort of the patients were divided into two groups depending on affiliation to a cluster with severe and moderate ischemia and severe and moderate macular edema. Herewith, Cluster 4 patients had both severe ischemia and severe macular edema and were included in both groups. Using FAG and OCT data and integral clinical burden assessment, ROC analyses was performed in the patients of all clusters and we found optimal dividing points for diagnosis of patients according to the disease character (the course of the disease). In the RVO patients, macular edema with the significant ischemic area was diagnosed when the optimal area value was > DD (ROC = 0.97; sensitivity, 92.6%; specificity 94.1%). Macular edema with severe foveal edema was diagnosed when the optimal foveal thickness value was > 574 µm (ROC = 0.99; sensitivity, 95.45%; specificity 94.9%). Integral clinical burden (a sum of clinical symptoms) was assessed as high when the optimal dividing point was > 8 scores (ROC = 0.91; sensitivity, 77.8%; specificity 85.3%). Thus, criteria of RVO-associated macular edema severity are: • FAG area of the ischemic zone > 5 DD • OCT fovea thickness > 574 µm • Integral clinical burden assessment > 8 scores. At the following stage of the study, we tested a hypothesis that the revealed features of the macular edema course could have an effect on laser treatment outcomes performing threshold and subthreshold laser retinal photocoagulation. A value of changes in VA, presented in octaves and considering baseline and end-point VA, was used as a response to treatment [2, 4]. Table 4 presents the treatment outcomes data for the RVO-associated ME patients depending on a laser photocoagulation type and the criteria of macular edema severity. Laser treatment outcomes given in Table 4 evidence that treatment success of threshold and subthreshold laser photocoagulation for RVO-associated ME was different depending on the macular edema severity. Thus, based on Table 3 data, subthreshold laser photocoagulation was more successful than threshold laser photocoagulation in the patients with apparent retinal ischemia (р = 0.026) while threshold laser photocoagulation was more successful than subthreshold laser photocoagulation in the patients with severe foveal edema, as considering VA improvement. In high clinical burden, subthreshold laser photocoagulation also had an advantage of threshold laser photocoagulation; however, data obtained were not statistically significant. This result could be caused by the fact that high clinical burden was usually combined with the pronounced ischemic area (Table 2) which subthreshold laser photocoagulation was proved to be more successful in.
Based on the assessment of macular edema severity, the most successful laser treatment for the patients with low macular edema severity values was subthreshold laser photocoagulation. The upper part of Table 3 presents the results of treatment for patients with high macular edema severity values accompanied by a significant VA decrease. The VA changes at baseline and after treatment (given in octaves) were greater in such patients than in the patients with less VA impairment. An increase in VA by 1 octave corresponded to a two times increase of VA. Data given in Table 4 demonstrate that, in the patients with the large ischemic area, subthreshold laser photocoagulation resulted in the VA increase that statistically significantly exceeded the VA increase in the patients undergone threshold laser photocoagulation (Р = 0.045). However, in the patients with the high foveal thickness, the VA increase was significantly greater after threshold laser photocoagulation compared to VA after subthreshold laser photocoagulation that did not reach one octave. In the patients with low severity values (the lower part of Table 4), the efficacy of subthreshold laser pho-tocoagulation was significantly higher in the patients with the ischemic area of < 5 DD (р = 0.049). The sum of clinical symptoms < 8 scores was also accompanied by the insignificant ischemic area, and this can explain the more successful subthreshold laser photocoagulation in these patients.
High clinical burden can be an evidence of the decreased capability of the retina to compensate negative consequences of RVO and maladaptation of the retina to the changed blood flow conditions. The difference in distribution of the clinical symptoms in clusters can point at two inter-related factors: a stage of edema development and retinal adaptation to the new blood flow conditions. These conclusions are based on revealing the occurrence of new symptoms and the disappearance of previously detected symptoms in the fundus of the patients in different clusters. Such symptoms as neovascularization and hard exudates appear later than all other symptoms while soft exudates and intraretinal hemorrhages are the first to disappear [11]. It is known that hard exudates are common for acute vascular retinal diseases and neovascularization is formed at a later time term, from 3 to 12 months. This is very important for making up a therapeutic decision since the occurrence of microaneurysm and neovasularization in the retina leads to secondary edema development and laser photocoagulation of these formations is more conditioned for such patients than vascular endothelium growth factor inhibitors which do not have an effect on the present so far microaneurysms [7, 14]. The findings on subthreshold laser photocoagulation to hold the advantage in most clinical groups are confirmed with our previous studies where we have shown that subthreshold laser retinal photocoagulation is more effective for RVO-associated ME treatment as compared to threshold laser photocoagulation [6]. Conclusions Based on cluster analysis and using automatic grouping, we defined four clusters of patients, maximally different in several features at the same time, both morphometrical and due to integral clinical symptoms assessment. This mathematical classification made it possible to reduce the heterogeneity of the studied cohort since the defined clusters were homogenous in severity criteria; the patients had similar values within each cluster. Based on the search for the optimal dividing points using ROC analysis, we developed a diagnostic model for the cluster assignment of any new patient according to macular edema severity. Treatment outcomes for two methods of laser photocoagulation were consistent with the developed assessment of macular edema severity. Subthreshold photocoagulation was best for the significant ischemic area (>5 DD). For the foveal thickness >547 µm, most successful was threshold laser photocoagulation. When the patients had both the significant retinal ischemia and the increased foveal thickness, subthreshold laser photocoagulation was more successful as well. In the patients with moderate disorders with the low severity values, the best success was achieved using threshold laser photocoagulation. References
|