J.ophthalmol.(Ukraine).2017;5:34-38.
|
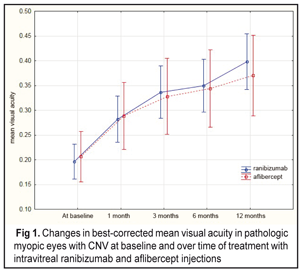
https://doi.org/10.31288/oftalmolzh201753438 Comparison of intravitreal ranibizumab and aflibercept in patients with choroidal neovascularization in pathologic myopia О.M. Blavatska1, T.B. Kustryn2, A.R. Korol2, Dr Med Sc 1Danylo Halytsky Lviv National Medical University 2Filatov Institute of Eye Disease and Tissue Therapy, Odessa E-mail: syannya@rambler.ru, laserfilatova@gmail.com Purpose. To compare the efficacy of ranibizumab and aflibercept treatment of patients with choroidal neovascularization (CNV) in pathologic myopia. Material and Methods. This was a comparative, uncontrolled, prospective cohort study, enrolling 96 pathologic myopic eyes (93 patients) with subretinal neovascularization. Results. 65 eyes (63 patients) were treated with 0.5 mg intravitreal ranibizumab; 31 eyes (30 patients) were treated by 2 mg intravitreal aflibercept. The baseline mean visual acuity was 0.2 and 0.21 in the intravitreal ranibizumab (IVR) and intravitreal aflibercept (IVA) groups, respectively. At 12 months, the mean visual acuity was 0.4 and 0.37 for the IVR and IVA groups, respectively. The mean CRT at baseline was 314.6 µm and 276.5 µm for the IVR and IVA groups, respectively. At 12 months, the mean CRT was 226.7 µm (р = 0.000) and 224.3 µm (р = 0.000) for the IVR and IVA groups, respectively. The mean number of injections in the IVR and IVA groups was 2.3 (0.9) and 2.6 (0.9), respectively. No statistically significant difference was noted in the number of injections between groups (р = 0.15). Conclusions. Both ranibisumab and aflibercept were found to improve the visual acuity in pathologic myopic patients with CNV with no statistically significant difference in the number of intravitreal injections. Key-words: pathologic myopia, choroidal neovascularization, ranibisumab and aflibercept Introduction The incidence of myopia varies within a wide range depending on a geographic area, nationality, and age. So, pathologic myopia, defined as a spherical equivalent higher than 6 diopters, is revealed in 6% to 18% of cases among all myopic subjects. It has been reported to be 0.5% to 2% among population of the USA and Europe [1]. The problem is of a certain importance since it can lead to irreversible impairment of visual functions. Among persons at the age of 50 and below, myopia is a cause of subretinal neovascularization (CNV) in 62% [7]. Myopia takes the second place following age-related macular degeneration (AMD) among CNV-complicated nosologies that lead to central vision loss [2]. In 2003, Ohno-Matsui et al reported that 35% of cases when a myopic eye developed CNV, the latter was developed in a fellow eye within eight years [16]. Laser coagulation, transpupillary thermotherapy, and photodynamic therapy have been proposed for CNV treatment. It should be noted that although laser treatments are rather efficacious, long-term results of these treatments are often poor [1, 2, 17, 24]. Many diseases of the macula are widely treated using inhibitors of vascular endothelial growth factor (VEGF), ranibizumab and aflibercept particularly. Thus, randomized clinical trials have reported on their efficacy for AMD of the retina [4, 9, 20], for macular edema with retinal central vein occlusion [3, 5, 10, 15], and for diabetic macular edema [11, 14]. International multicenter studies (RADIANCE, REPAIR) have shown the efficacy and safety of ranibizumab for treatment of myopic CNV [22, 23, 25]. Also, the MYRROR study has reported on the efficacy of aflibercept for the treatment of patients with myopic CNV [27]. The studies mentioned above have been conducted independently of each other, however simultaneous comparison of the angiogenesis therapy in patients with pathologic myopia complicated by CNV have not been performed. To-date, a worldwide current issue is one on a choice of treatment that would be most efficacious for patients with myopic CNV with an optimal regimen of intravitreal anti-VEGF injections. The purpose of the study was to compare the efficacy of ranibizumab and aflibercept treatment of patients with subretinal neovascularization in pathologic myopia. Material and Methods This was a comparative, uncontrolled, prospective cohort study, enrolling 96 pathologic myopic eyes (93 patients) with subretinal neovascularization. The study was carried out at Ocular Laser Microsurgery Department of Filatov Institute of Eye Diseases and Tissue Therapy and at Ophthalmology Department of Danylo Halytsky Lviv National Medical University. Before the initiation of the study, all patients were obtained a written informed consent approved by a local scientific ethical committee according to Helsinki Declaration principles. Inclusion criteria for this study were the presence of CNV with pathologic myopia, spherical equivalent of myopia ?6.0 diopters (if spherical equivalent of myopia is <6.0 diopters, a patient must have macular alterations, typical for shortsightedness: lacquer cracks, choroidal atrophy, posterior staphyloma), axial length ?26.5 mm, recent (within two months) history of CNV associated with pathologic myopia. Patients were divided into two groups depending on the drug injected. The first group patients were treated with 0.05 ml (0.5 mg) intravitreal ranibizumab and the second group patients were treated with 0.05 ml (0.2 mg) intravitreal aflibercept. The patients were followed up over 12 months. We used a pro re nata (PRN) dosing strategy with two compulsory monthly loading doses; the further injections were performed as required and depended on the worsening of visual acuity, ophthalmoscopically revealed subretinal hemorrhage, the OCT thickening of the macula and CNV, and hyperfluorescent in the macula. Each patient underwent best-corrected visual acuity (BCVA) assessment, refractometry, axial length (AL) measurement, biomicroscopy, macular optical coherence tomography (OCT), and fluorescein fundus angiography (FFA). FFA was performed at baseline and on completion of the treatment. No hypofluorescence at treatment completion was considered as closure of neovascularization. The presence of hypofluorescence on FFA evidenced of active CNV. A primary end point was the best corrected visual acuity (BCVA) at 12 month of treatment. Secondary end points were OCT central retinal thickness (CRT), activity of CNV as evaluated by fluorescein angiography, the number of intravitreal injections, and safety of the drugs. Primary data base was developed, the data were statistically processed, and diagrams were designed using Statistica® 10.0 (StatSoft©). To characterize the groups, the mean (M) and standard deviation (SD) were computed. When studying changes in measurements (BCVA, CRT, and CNV) in the groups after intravitreal injections, we used repeated measures analysis of variance. Mann-Whitney criterion was used to compare groups. A p-value of <0.05 was accepted as a statistically significant level in all analyses. Results The age of patients averaged 47 years; 96.8% aged 30 to 70 years and 83% of patients were women and 17% were men. Refraction values ranged from –6.0D to –24.5D. Whereas, refraction of –25D to –20D was recorded in 11 eyes (11%), –20D to –15D in 23 eyes (24%), –15D to –10D in 32 eyes (33%), and –10D to –5D in 30 eyes (31%). Axial length of the eye varied within the range between 23.1 mm and 32.6 mm. Whereas, the values of AL of 26 mm to 28 mm was noted in 38 eyes (39.6%), 28 mm to 30 mm in 41 eyes (42.7%), 30 mm to 32 mm in 13 eyes (13.5%), >32 mm in 4 eyes (4.2%). The median AL value was 28.4 mm. All patients were divided into two groups: group 1 comprised 65 eyes (63 patients) treated with intravitreal ranibisumab (IVR); group 2 comprised 31 eyes (30 patients) treated with intravitreal aflibercept (IVA). The mean BCVA at baseline was 0.2 (0.14) and 0.21 (0.15) in the IVR and IVA groups, respectively. No statistically significant difference was noted between the two groups (р=0.89). Over the follow-up period, the BCVA significantly increased in both groups: at month 12, the mean BCVA was 0.4 (р=0.000) and 0.37 in the IVR and IVA groups, respectively (Fig. 1).
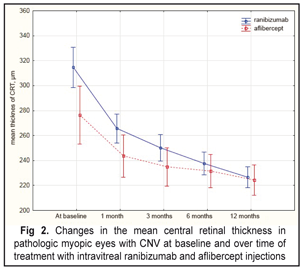
The Mann-Whitney criterion was used to compare the mean BCVA at each follow-up point. However, no difference in the mean BCVA was noted between the patients’ group studied. The mean baseline CRT was 314.6 µm and 276.5 µm in the IVR and IVA groups, respectively. And the mean CRT differed significantly between the two groups (р=0.000). However, at the following time points, the difference between the IVR and IVA groups was not statistically significant for this parameter. Over the whole follow-up period, a decrease in CRT was noted in both groups. Thus, at month 12 the mean CRT significantly decreased by 28% as compared to baseline, i.e. from 314.6 µm to 226.7 µm, in the IVR group. However, in the IVA group, OCT also revealed a significant decrease in CRT at each follow-up points (р=0.000). At 12 months after the initiation of treatment, the mean CRT in the IVA group significantly decreased by 18.9% compared to baseline, i.e. from 276.5 µm to 224.3 µm (Fig. 2). The difference in CRT between the IVR and IVA groups was statistically insignificant (р=0.3).
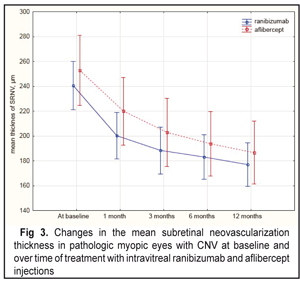
At baseline, the mean CNV thickness was 240.6 µm and 252.7 µm in the IVR and IVA, respectively. However, the mean CNV thickness did not differ significantly between two groups (р=0.46). A significant decrease in the CNV thickness was noted in the both groups studied. Thus, on completion of the follow-up, the mean CNV decreased statistically significantly by 26.5% as compared to baseline, i.e. from 240.6 µm to 176.9 µm, in the IVR group. In the IVA group, at month 12, compared to baseline, the mean CNV also decreased statistically significantly by 26.2%, i.e. from 252.7 µm to 186.6 µm. At each follow-up point, the differences in the CNV values were insignificant (Fig. 3).
On average, the mean number of injections was 2.3 (0.9) and 2.6 (0.9) in the IVR and IVA groups. No statistically significant difference in the number of injections was noted between the groups (р = 0.15). No case of endophthalmitis, uveitis, retinal detachment, or cardiovascular sequelae was noted. Discussion The efficacy of ranibisumab and aflibercept has been studied separately in the previous investigations. Ranibisumab has been shown to improve functional and anatomical measures in pathologic myopic patients with RSNV in a number of prospective cohort studies (from 9 to 64 patients) [12, 13, 18, 21, 26] in addition to a double-masked randomized clinical study RADIANCE (227 patients) and an open-label uncontrolled study REPAIR [22, 23]. Thus, the RADIANCE and REPAIR studies have proven that ranibisumab improves vision acuity in patients with myopic CNV, on average, by 14 letters following two intravitreal ranibisumab injections with follow-up of 12 months. These two studies recorded similar changes in the mean retinal thickness at the fovea. So, RADIANCE has reported on a reduction in the mean CRT of 71.3 µm at month 12 of the treatment [25]. A number of small prospective and retrospective studies have also demonstrated positive functional and structural results of treatment of pathologic myopic patients with CNV using ranibisumab. For instance, Lai TY et al have shown the improvement of the visual acuity, on average, by 3 lines in 16 patients with myopic CNV in pathologic myopia with a 12 month treatment course. Ranibisumab resulted in a significant reduction of the OCT mean central retinal thickness [12]. Mones et al have demonstrated a significant increase in the mean visual acuity by 9.53 letters with the mean number of intravitreal ranibisumab injections of 1.52 over 12 months [13]. Silva et al have determined that, at 12 months follow-up, visual acuity in patients with myopic CNV improved, on average, from 20/100 to 20/63 following 3.6 intravitreal ranibisumab injections [21]. Wu et al have studied the results of three loading sequential monthly intravitreal ranibisumab injections followed by a PRN schedule in myopic CNV. Herewith, the mean visual acuity was significantly increased over 12 month treatment with a mean injection number of 3.44 (SD 0.92) [26]. Cha et al have reported on results of studying the efficacy of ranibisumab (22 patients) in comparison with bevacizumab (42 patients) in myopic CNV at 12 months follow-up. The mean visual acuity significantly increased in both groups. And the mean central retinal thickness significantly decreased by 22.43% and 15.56% in the ranibisumab and bevacizumab groups, respectively. The mean number of injections over the follow-up period was 2.43±1.04 and 2.72±0.96 in the ranibisumab and bevacizumab groups, respectively (р=0.27) [6]. In our study, in the IVR group we noted a significant improvement of the mean visual acuity, a decrease in the mean central retinal thickness at the fovea and in the CNV thickness following two intravitreal ranibisumab injections at 12 month follow-up. In 2015, there appeared results of the MYRROR study on intravitreal aflibercept for myopic CNV. Aflibercept treatment resulted in the improvement of the mean visual acuity, on average, by 13.5 letters at 48 week follow-up [27]. In this study, the mean CRT significantly decreased from 349.7 µm to 263.5 µm. The mean number of injections was 2 (at the first quarter of the study) and 0 (from the second to fourth quarters of the study) [27]. Pece et al have reported the results of treatment of 33 patients with myopic CNV. At 12 months, compared to baseline the mean visual acuity did not increase statistically significantly, the mean CRT significantly decreased from 242±56 µm до 198±46 µm (р=0.02), and the mean number of injections was 2.0 (1 to 4 injections) [19]. In the IVA group we noted a significant decrease in the mean BCVA and a reduction of the mean CRT and of the CNV thickness at 12 month follow-up. Over the follow-up period the mean number of injections was 2.6. Limitations in our study were: difficulties in comparing the finding of our study with other studies because of using different visual acuity charts; using different OCT units; different follow-up periods. Our study demonstrated that in clinical practice both ranibisumab and aflibercept could increase the visual acuity and improve anatomic and functional measures in pathologic myopic patients with CNV over 12 months. Conclusions
Both ranibisumab and aflibercept were found to improve the visual acuity in pathologic myopic patients with CNV with no statistically significant difference in the number of intravitreal injections. There is no difference between ranibisumab and aflibercept to be used in the clinical practice. References 1.Medvedev IB, Belikova EI, Syamichev M.P. [Photodynamic therapy in ophthalmology]. OOO “GUPT “Krasnyi voin””; 2006. 152 p. In Russian. 2.Blinder KJ, Blumenkranz MS, Bressler NM, et al. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial FVIP report no. 3. Ophthalmology. 2003; 110:667–673. 3.Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117:1124-1133. 4.Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009; 116:57-65. 5.Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010; 117:1102-1112. 6.Cha DM, Kim TW, Heo JW, et al. Comparison of 1-year therapeutic effect of ranibizumab and bevacizumab for myopic choroidal neovascularization: a retrospective, multicenter, comparative study.BMC Ophthalmology. 2014; 14:69. 7.Cohen SY, Laroche A, Leguen Y, et al. Etiology of choroidal neovascularization in young patients. Ophthalmology. 1996; 103:1241-1244. 8.Cornut PL , Poli M , Feldman A, et al. Intravitreal ranibizumab for choroidal neovascularization secondary to pathological myopia: 12-month results. J Fr Ophtalmology. 2010; 33(5):327-333. 9.Heier JS, Brown DM, Chong V et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology.2012; 119:2537-2548. 10.Heier JS, Clark WL, Boyer DS et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology. 2014; 121:1414-1420. 11.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology.2014; 121:2247-2254. 12.Lai TY, Chan WM, Liu DT, et al. Intravitreal ranibizumab for the primary treatment of choroidal neovascularization secondary to pathologic myopia. Retina. 2009; 29:750-756. 13.Mon?s JM, Amselem L, Serrano A, et al. Intravitreal ranibizumab for choroidal neovascularization secondary to pathologic myopia: 12-month results. Eye (Lond). 2009; 23(6):1275-1280. 14.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012; 119:789-801. 15.Ogura Y, Roider J, Korobelnik JF, et al. Intravitreal aflibercept for macular edema secondary to central retinal vein occlusion: 18-month results of the phase 3 GALILEO study. Am J Ophthalmol.2014; 158:1032-1038. 16.Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol.2003;87:570-573. 17.Ozdek S, Hondur A, Gurelik G, et al. Transpupillary thermotherapy for myopic choroidal neovascularization: 1-year follow-up International Ophthalmology. 2005; 26(4-5):127-133. 18.Pasyechnikova NV, Naumenko VO, Korol AR, et al. Intravitreal ranibizumab for the treatment of choroidal neovascularizations associated with pathologic myopia: a prospective study. Ophthalmologica. 2015; 233(1):2-7. 19.Pece A, Milani P. Intravitreal aflibercept for myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2016 Dec; 254 (12):2327-32. 20.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419-1431. 21.Silva RM, Ruiz-Moreno JM, Rosa P, et al. Intravitreal ranibizumab for myopic choroidal neovascularization: 12-month results. Retina. 2010; 30: 407-412. 22.Tufail A, Narendran N, Patel PJ, et al. Ranibizumab in myopic choroidal neovascularization: the 12-month results from the REPAIR study. Ophthalmology. 2013; 120:1944-1945. 23.Tufail A, Patel PJ, Sivaprasad S, et al. Ranibizumab for the treatment of choroidal neovascularisation secondary to pathological myopia: interim analysis of the REPAIR study. Eye (Lond).2013; 27:709-715. 24.Virgili G, Menchini F. Laser photocoagulation for choroidal neovascularization in pathologic myopia. Cochrane Database Syst Rev.2005; (4):CD004765. 25.Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology.2014;121:682-692. 26.Wu TT, Kung YH. The 12-month outcome of three consecutive monthly intravitreal injections of ranibizumab for myopic choroidal neovascularization. J Ocul Pharmacol Ther. 2012; 28(2):129-133. 27.Yasushi Ikuno, Kyoko Ohno-Matsui, Tien Yin Wong, et al. Intravitreal Aflibercept Injection in Patients with Myopic Choroidal Neovascularization The MYRROR Study. Ophthalmology.2015; 122: 1220-1227.
|