J.ophthalmol.(Ukraine).2017;5:26-33.
|
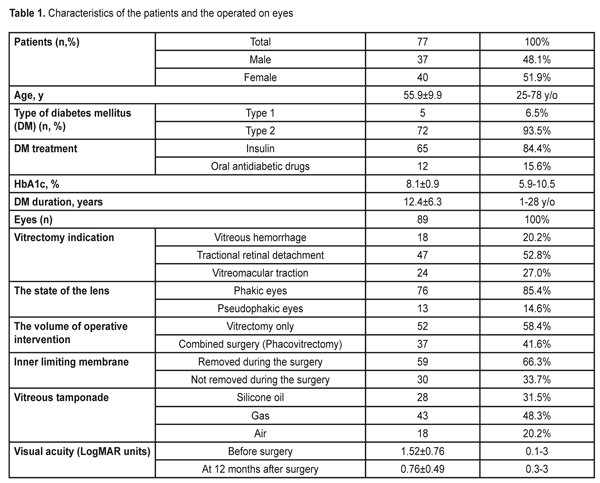
https://doi.org/10.31288/oftalmolzh201752633 Changes in the retinal thickness after microinvasive vitrectomy with inner limiting membrane removal in patients with diabetic retinopathy M.I.Kerimov National Centre of Ophthalmology named after acad. Zarifa Aliyeva Baku (Azerbaijan) E-mail: mushfikk@mail.ru The purpose. To study changes in the macular retinal thickness in patients with diabetic retinopathy after microinvasive vitrectomy with inner limiting membrane (ILM) removal. Material and Methods. This was a retrospective analysis of spectral OCT data of the macula of eighty-eight eyes (77 patients) after 23 gauge vitrectomy for proliferative diabetic retinopathy. The surgeries were performed at National Centre of Ophthalmology named after acad. Zarifa Aliyeva, Baku, Azerbaijan, in the period between 2010 and 2015. Study group comprised fifty-nine eyes in which vitrectomy was performed with ILM removal; Control group comprised thirty eyes in which ILM was not removed during the surgery. ILM was peeled using ILM forceps (Alcon, the USA) after brilliant blue G (BBG) staining. For statistical analysis, we used three Cirrus-HD OCT measurements: central (1 mm), inner temporal (1-3 mm), and inner nasal (1-3 mm) retinal thickness. Results. At 12 months after surgery, the mean macular thickness in the inner temporal subfield was much thinner in the eyes with ILM removal than in those without ILM removal, 279±51.9 ?m vs. 332±93.8 ?m, (p=0,002), respectively. The macular thickness in the central, foveolar subfield almost did not differ in different groups as well as the thickness in the inner nasal subfield (p>0.05 in both cases). Since there was not a healthy paired eye, we compared our data with the normative data obtained from the paper by Liu et al [2011], in which Cirrus HD OCT showed the macular thickness in the inner temporal subfield equal to 313.4±18.5 ?m in diabetic patients without retinopathy signs, which was significantly higher than in our patients after vitrectomy with ILM removal (295±43.1 ?m, p=0.002). And the macular thickness in the nasal and central foveal subfields did not differ significantly from our data. Conclusions. We revealed thinning of the macular temporal subfield after vitrectomy with ILM removal not only in patients with diabetic macular edema but also with tractional retinal detachment and vitreous hemorrhage. Our data confirm, once again, a universal character of the asymmetric changes in the macular thickness in the temporal and nasal subfields after vitrectomy with ILM removal for various pathological changes in the retina. However, to clarify the practical meaning of this phenomenon, the further functional investigations are required. Key-words: retinal thickness, vitrectomy with inner limiting membrane removal, diabetic retinopathy Background Diabetic retinopathy (DR) is one of the leading causes of low vision among working-age adults in the developed countries [1, 2]. Vitrectomy is an effective treatment method for such DR complications as nonclearing vitreous hemorrhage, tractional retinal detachment, vitreomacular traction and others. Introducing a microinvasive 23-27 gauge technique into the clinical practice over the last decades, improving technical characteristics of surgical systems for vitrectomy, and using various supporting tools, in particular, vital dyes in the vitreoretinal surgery (chromovitrectomy), make it possible to improve outcomes of the surgical treatment [3-6]. Originally, vitrectomy with the removal of the inner limiting membrane (ILM) has been performed for surgical treatment of idiopathic macular holes [7]. Later on, ILM peeling has come into use for treatment of epiretinal membrane (ERM), tractional diabetic macular edema (DME) etc. [8, 9]. In literature, there has been reported that ILM peeling can help in preventing epiretinal membrane development (re-proliferation) after vitrectomy for rhegmatogenous retinal detachment and diabetic tractional detachment [10-13]. At the same time, only since spectral coherent tomography (OCT) was introduced into the clinical practice, in-life observation over morphological changes in the retinal macula after vitrectomy, including those after ILM peeling, has become possible. Following up patients operated on for macular holes has made it possible to reveal such specific changes after ILM peeling as dissociated optic nerve fiber layer (DONFL) and temporal macula thinning [14]. Recently, there have been papers pointing similar changes in the retina after ILM removal in patients with epiretinal membranes (ERM) [15, 16]. To our best knowledge, there is only one paper on the changes in the retina with diabetic macular edema after vitrectomy with ILM removal [17]. The purpose of the present paper was to study changes in the macular retinal thickness in patients with diabetic retinopathy after microinvasive vitrectomy with inner limiting membrane removal. Material and Methods The present paper is a retrospective analysis of spectral OCT data of the macula of eighty-eight eyes (77 patients) after 23 gauge vitrectomy for proliferative diabetic retinopathy. The surgeries were performed at National Centre of Ophthalmology named after acad. Zarifa Aliyeva, Baku, Azerbaijan, in the period between 2010 and 2015. The study included only cases, in which anatomical success was achieved; the cases were followed up for twelve months and OCT study was performed. Study group comprised fifty-nine eyes in which vitrectomy was performed with ILM removal; Control group comprised thirty eyes in which ILM was not removed during the surgery. The study excluded the eyes in which silicone oil remained for more than six months. The main characteristics of the patients observed and the eyes operated on are given in the table 1.
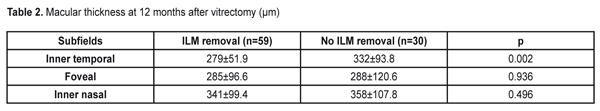
A surgery technique. Microinvasive 23 gauge pars plana vitrectomy was performed using Accurus 800CS and Constellation surgical systems (Alcon, the USA) under Lumera 700 and Lumera T surgical microscopes (Zeiss, Germany) and a viewing system B?OM 4m (Oculus, Germany) to visualize the anterior segment. When performing a combined surgery, cataract phacoemulsification and IOL implantation in the capsular bag was performed in the beginning of the surgery. In most cases, epiretinal membranes were removed with a vitreous cutter; scissors were rarely used. ILM was peeled using ILM forceps (Alcon, the USA) after brilliant blue G (BBG) staining; a micropick and a Tano scraper were not used. Optical Coherent Tomography (OCT) was performed using spectral OCT Cirrus-HD (Carl Zeiss Meditec, the USA). Two protocols, Macular cube 512 ? 128 with measurements of the retinal thickness in nine zones and 5-line HD raster, were used. For statistical analysis, we used measurements in three subfields: central (the diameter of the ring was 1 mm); inner temporal (the diameter of the ring was 1-3 mm); and inner nasal (the diameter of the ring was 1-3 mm). In doubtful cases, the retinal thickness was measured manually. Statistical Analysis. The data obtained were processed using SPSS statistic software (IBM SPSS Statistics, 20.0). The quantitative values were presented as mean±standard deviation. Student t-criterion was used to compare the mean values in different groups for independent samples and t-criterion was used to assess the dynamics of changes for paired samples. A level of significance was accepted as p=0.05, which corresponds to criteria accepted for medical investigations. If the p-value was lower than 0.001, it was pointed as p<0.001. The study was approved by Academic Council of National Centre of Ophthalmology named after acad. Zarifa Aliyeva. The informed consent was obtained from each patient. Results To determine the effect of ILM removal on the post-operative retinal thickness, we compared the retinal thickness in the inner temporal, central, and inner nasal subfields using data of spectral OCT at 12 months after microinvasive vitrectomy with and without ILM removal during the surgery for DR complications. As it can be seen in Table 2, at 12 months after surgery, the mean retinal thickness differed significantly in different groups only in the inner temporal subfield of the macula. Thus, temporal macula was much thinner (279±51.9 ?m) in the eyes where ILM was removed than in those without ILM removal, (332±93.8 ?m, p=0.002). The thickness in the central, foveolar subfield almost did not differ in different groups as well as the thickness in the inner nasal subfield (p>0.05 in both cases).
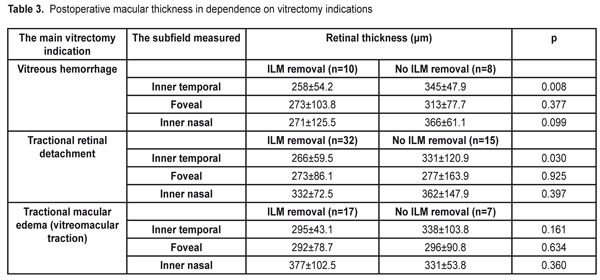
Besides, at 12 month after surgery, we compared the retinal thickness in groups depending on the vitrectomy indications: vitreous hemorrhage, tractional retinal detachment, and vitreomacular tration. The results are given in Table 3.
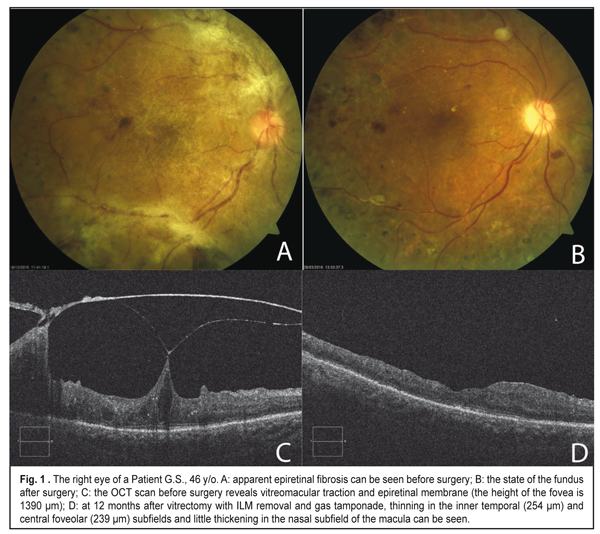
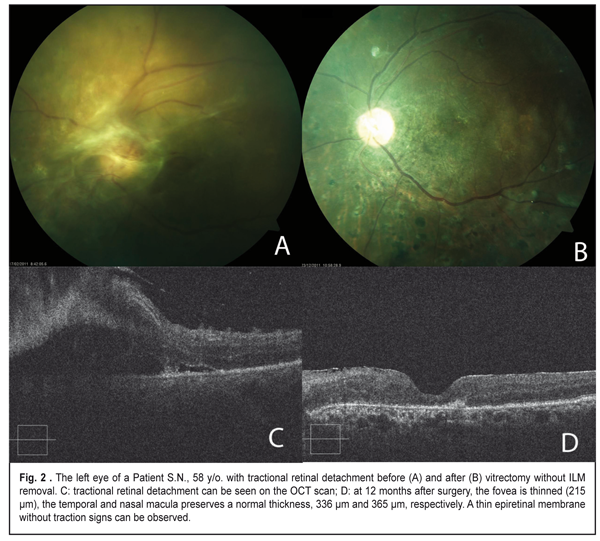
As it can be seen in Table 3, the retinal thickness at 12 months after surgery differed significantly between cases with and without ILM removal in all groups. In a group with vitreous hemorrhage, the mean retinal thickness in the temporal subfield of the inner ring was 258±54.2 ?m in the eyes with ILM removal vs. 345±47.9 ?m in the eyes where ILM was not removed (р=0.008). Of a lower rate were the values of the mean thickness in the central and nasal subfields in the group with the removed ILM; however the difference was not significant. The mean thickness in the temporal subfield was also significantly thinner after vitrectomy for tractional retinal detachment in the eyes with ILM removal as compared with eyes where ILM was not removed, 266±59.5 ?m and 331±120.9 ?m, respectively, (p=0.030). The thickness in the foveolar zone and in the inner nasal subfield little differed after vitrectomy with and without ILM removal in patients with tractional retinal detachment. In a group with tractional macular edema (vitreomaular traction), there were similar tendencies; however; the difference between groups was not statistically significant because of the lack of observational cases. OCT measurements of the macular area before surgery were possible to perform only in the eyes with vitreomacular traction (VMT). As it can be seen in Table 4, in a group with the removed ILM, all OCT values in the inner ring decreased at one year after surgery. So, the mean thickness in the inner temporal subfield of the macular before surgery was 464±97.7 ?m vs. 295±43.1 ?m at 12 months after surgery (p<0.001). The mean thickness decreased significantly, from 520±167.9 to 292±78.7 ?m in the foveolar subfield (p=0.001) and from 495±191.8 to 377±102.5 ?m in the inner nasal subfield of the macula (p=0.007). In seven eyes with VMT, where ILM was not removed, a decrease was significant only in the foveolar thickness, from 498±87.3 to 296±90.8 ?m (p=0.014); although other values were decreased, they, especially the temporal thickness, were not as significant as in the group with the removed ILM. Besides, because of the absence of a healthy paired eye, we also compared data on the macular thickness after vitrectomy with ILM removal with the normative data from the literature. Liu et al. [18] has reported on Cirrus HD OCT data that, in diabetic persons without retinopathy signs, the mean macular thickness in the inner temporal subfield is 313.4±18.5 ?m, which is significantly higher than in our patients after vitrectomy with ILM removal, which was 295±43.1 ?m (p=0.002). And the macular thickness in the nasal and central foveolar subfields do not differ significantly from our data, 315.9±18.8 ?m vs. 341±99.4 ?m (p=0.180) and 258.5±21.6 ?m vs. 285±96.6 ?m (p=0.251), respectively. Figure 1 demonstrates an eye of a patient with vitreomacular traction and extramacular tractional detachment before and after 23 gauge vitrectomy with ILM removal; and Figure 2 demonstrate an eye of a patient with tractional retinal detachment before and after vitrectomy without ILM removal.
Discussion Vitrectomy with ILM removal was originally proposed for macular holes [7], and, later on, for other pathologic changes of the vitreomacular interface including idiopathic epiretinal membranes, tractional diabetic macular edema and others [8, 9, 19]. Improvements and innovations in the diagnostic equipment, in particular, the advent of OCT, have made it possible to reveal specific changes in the inner retinal layers occurring after ILM removal in patients with macular pathology. First, Tadayoni has noted changes in the nerve fiber layer, which he called dissociated optic nerve fiber layer (DONFL), in the eyes with macular holes after removing the ILM [14]. These changes, according to authors, were noted at 1-3 months after surgery and were not associated with functional changes according to findings of visual acuity test, visual field test, and microperimetry [20, 21]. Other changes having been detected after ILM removal in the eyes with macular holes are thinning of the temporal part and thickening of nasal part of the macula, numerous dimples on the retina surface, displacement of the fovea to the optic disc, paracentral macular holes [22-25]. Later on, similar changes have been described for patients with ERM, whereby the authors have concluded that the pointed changes in the retina after ILM removal can be of a universal character [15, 26, 27]. Although the expirience of ILM removal in surgery for diabetic macular edema is sufficient, there are a few reports on postoperative changes in the retina according to OCT findings. To the best of our knowlege, there is a single paper by Yoshikawa et al. [17], in which the authors have paid more attention to displacement of the fovea toward the optic disc than to changes in the retinal thickness in eyes with ILM peeling for DME. We revealed thinning of the temporal subfield and thickening of the macula in patients with ILM peeled for various DR complications. Contrary to the previous papers on macular holes and ERM, in which authors could use a healthy paired eye for comparison, we could not do it because of severe PRD-associated structural changes in the paired eyes. That’s why we compared our findings on the retinal thickness with those for the diabetic eyes without diabetic retinopathy signs, obtained from the literature [18]; herewith, the most significant data were obtained for the macular thickness in the temporal subfield. The macular thickness in the temporal subfield after vitrectomy with ILM removal in our study was 295±43.1 ?m, which is significantly lower that the normative data (313.4±18.5 ?m). It should be noted that we revealed the thinning of the temporal macula after vitrectomy with ILM removal not only in patients with DME but in those with tractional retinal detachment and vitreous hemorrhage, which we did not come across in the literature. Yoshikawa et al. [17] believes that displacement of the fovea to the optic disc in DME patients after vitrectomy with ILM peeling leads also to the thinning of the temporal subfield and the thickening of the nasal subfield of the macula. According to the authors, after the ILM, which is a rigid basal membrane, is removed, other mechanical forces, in particular, axonal contractility, can be a cause of the shortening of papillofoveal distance and the appearing of asymmetric changes in the macular thickness.
Our data confirm once again the universal character of the asymmetric changes in the macular thickness in temporal and nasal subfields after vitrectomy with ILM removal for various pathological changes in the retina. However, to clarify the practical meaning of this phenomenon, the further functional investigations are required. References 1. Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556–564. 2. Scanlon PH, Aldington SJ, Stratton IM. Epidemiological issues in diabetic retinopathy. Middle East Afr J Ophthalmol. 2013 Oct-Dec;20(4):293-300. 3. Couch SM, Sophie J Bakri SJ. Use of triamcinolone during vitrectomy surgery to visualize membranes and vitreous. Clin Ophthalmol. 2008 Dec; 2(4): 891–896. 4. Park DH, Shin JP, Kim SY. Comparison of clinical outcomes between 23-gauge and 20-gauge vitrectomy in patients with proliferative diabetic retinopathy. Retina. 2010;30:1662–70. 5. Schoenberger SD, Miller DM, Riemann CD, et al. Outcomes of 25-gauge pars plana vitrectomy in the surgical management of proliferative diabetic retinopathy. Ophthalmol Surg Laser Imag- ing. 2011;42(6):474–80. 6. Hern?ndez F, Alpizar-Alvarez N, Wu L. Chromovitrectomy: an Update. J Ophthalmic Vis Res. 2014 Apr; 9(2): 251–259. 7. Mester V, Kuhn F. Internal limiting membrane removal in the management of full-thickness macular holes. Am J Ophthalmol. 2000;129:767-777. 8. Park DW, Dugel PU, Garda J, et al. Macular pucker removal with and without internal limiting membrane peeling: pilot study. Ophthalmology. 2003;110:62-64. 9. Kwok AK, Lai TY, Li WW, et al. Indocyanine green-assisted internal limiting membrane removal in epiretinal membrane surgery: a clinical and histologic study. Am J Ophthalmol. 2004;138:194-199. 10. Rao RC, Blinder KJ, Smith BT, Shah GK. Internal limiting membrane peeling for primary rhegmatogenous retinal detachment repair. Ophthalmology. 2013 May;120(5):1102-3.e1-2. 11. Nam KY, Kim JY. Effect of internal limiting membrane peeling on the development of epiretinal membrane after pars plana vitrectomy for primary rhegmatogenous retinal detachment. Retina. 2015 May;35(5):880-5. 12. Akiyama K, Fujinami K, Watanabe K, Tsunoda K, Noda T. Internal Limiting Membrane Peeling to Prevent Post-vitrectomy Epiretinal Membrane Development in Retinal Detachment. Am J Ophthalmol. 2016 Nov;171:1-10. 13. Michalewska Z, Bednarski M, Michalewski J, Jerzy N. The role of ILM peeling in vitreous surgery for proliferative diabetic retinopathy complications. Ophthalmic Surg Lasers Imaging Retina. 2013 May-Jun;44(3):238-42. 14. Tadayoni R, Paques M, Massin P, et al. Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology 2001;108: 2279–2283. 15. Kumagai K, Ogino N, Furukawa M, Hangai M, Kazama S, Nishigaki S, Larson E. Retinal thickness after vitrectomy and internal limiting membrane peeling for macular hole and epiretinal membrane. Clin Ophthalmol. 2012;6:679-88. 16. Pichi F, Lembo A, Morara M, Veronese C, Alkabes M, Nucci P, Ciardella AP. Early and late inner retinal changes after inner limiting membrane peeling. Int Ophthalmol. 2014 Apr;34(2):437-46. 17. Yoshikawa M, Murakami T, Nishijima K, Uji A, Ogino K, Horii T, Yoshimura N. Macular migration toward the optic disc after inner limiting membrane peeling for diabetic macular edema. Invest Ophthalmol Vis Sci. 2013 Jan 21;54(1):629-35. 18. Liu T, Hu AY, Kaines A, Yu F, Schwartz SD, Hubschman JP. A pilot study of normative data for macular thickness and volume measurements using cirrus high-definition optical coherence tomography. Retina. 2011 Oct;31(9):1944-50. 19. Gandorfer A, Messmer EM, Ulbig MW, Kampik A. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina 2000; 20 (2):126–133. 20. Mitamura Y, Suzuki T, Kinoshita T, N. Miyano N, Tashimo A, Ohtsuka K. Optical coherence tomographic findings of dissociated optic nerve fiber layer appearance American Journal of Ophthalmology, 2004 vol. 137, no. 6, pp. 1155–1156. 21. Ito Y, Terasaki H, Takahashi A, Yamakoshi T, Kondo M, Nakamura M. “Dissociated optic nerve fiber layer appearance after internal limiting membrane peeling for idiopathic macular holes,” Ophthalmology 2005, vol. 112, no. 8, pp. 1415–1420. ? 22. Rubinstein A, Bates R, Benjamin L, Shaikh A. Iatrogenic eccentric full thickness macular holes following vitrectomy with ILM peeling for idiopathic macular holes. Eye (Lond). 2005 Dec;19(12):1333-5. 23. Ohta K, Sato A, Fukui E. Asymmetrical thickness of parafoveal retina around surgically closed macular hole. Br J Ophthalmol. 2010;94:1545–1546. 24. Sandali O, El Sanharawi M, Basli E, Lecuen N, Bonnel S, Borderie V, Laroche L, Monin C. Paracentral retinal holes occurring after macular surgery: incidence, clinical features, and evolution. Graefes Arch Clin Exp Ophthalmol. 2012 Aug;250(8):1137-42. 25. Kawano K, Ito Y, Kondo M, Ishikawa K, Kachi S, Ueno S, Iguchi Y, Terasaki H. Displacement of foveal area toward optic disc after macular hole surgery with internal limiting membrane peeling. Eye (Lond). 2013 Jul;27(7):871-7. 26. Mason JO 3rd, Feist RM, Albert MA Jr. Eccentric macular holes after vitrectomy with peeling of epimacular proliferation. Retina. 2007 Jan;27(1):45-8. 27. Rush RB, Simunovic MP, Aragon AV 2nd, Ysasaga JE. Postoperative macular hole formation after vitrectomy with internal limiting membrane peeling for the treatment of epiretinal membrane. Retina. 2014 May;34(5):890-6.
|