J.ophthalmol.(Ukraine).2017;5:21-25.
|
https://doi.org/10.31288/oftalmolzh201752125 Changes in incidence rates of retinopathy of prematurity in Odessa region in the years of 2009 to 2016 S.V. Katsan, Cand Sc (Med), E.S. Zaichko, a Postgraduate student SI “Filatov Institute of Eye Diseases and Tissue Therapy of NAMS of Ukraine" Odessa (Ukraine) E-mail: k.s.zaichko@gmail.com Background. Although retinopathy of prematurity (ROP) is a wide-spread disease, statistical data are absent; so studying the incidence of ROP in Ukraine is of importance. Purpose. To study the incidence of retinopathy of prematurity in Odessa oblast (region) in children whose birth weights were < 2500 g. Material and Methods. We followed up 2682 premature babies. The initial examination was performed at four to six weeks after children’s birth. The examination included ophthalmoscopy under medical mydriasis using a binocular forehead ophthalmoscope with aspheric lenses of 20 and 30 D. The examination findings were registered according to recommendations of the International Committee for Retinopathy of Prematurity and following the International Classification of Retinopathy of Prematurity (ICROP). Analysis was processed using softwares MedCalc v.16.8.4 (MedCalc SoftWare bvba, 1993-2016) and MedStat (Lyakh Yu.E., Gurianov V.G. 2004 – 2011). Results. The mean values for the period of 2009-2016 were 24.7% (95% CI: 18.2- 31.7%) for ROP incidence rates; 19.4% (95% CI: 13.6-25.9%) for stage 1-2 ROP; 4.9% (95% CI: 3.3-6.8%) for ROP cases requiring laser treatment. The stage 1-2 ROP incidence was 47.6% and 87.9% in 2009 and 2016, respectively (p<0.001 according to the Chi-squared test for the presence of the trend for the ordered categories). Conclusions. The incidence of retinopathy of prematurity increased in Odessa Oblast from 10% in 2009 to 27% in 2016. The incidence of stage 1-2 ROP increased from 4.7 % in 2009 to 23.8 % in 2016. The incidence of stage 3 ROP decreased to 2.3% in 2016 vs. 5.2% in 2009. In 2016, aggressive posterior ROP was diagnosed in 1.0 % of cases. The frequency of laser treatment decreased to 3.3% in 2016 compared to 5.2% in 2009. Key-words: retinopathy of prematurity, demographic situation
Background Retinopathy of prematurity (ROP) is one of the leading causes of blindness and visual impairment in children and is characterized by anomalous vascular development of the retina in premature babies. Over the last decade, the ROP-associated blindness incidence has increased in middle- and low-income countries where neonatal care for premature babies is improving as survival rates of infants with extremely low birth weight increase, which has started “the third epidemic” of the disease. According to data of World Health Organization (WHO), ROP remains one of the most important medical and social problems. It has been defined that 1,494 million children are blind or have visual impairment. Among them, 13 % are due to retinopathy of prematurity [4, 8, 16]. The incidence of the disease varies in different countries within the ranges of 15-36.5% of all children at high risk [3, 5, 12, 13]. However, it is difficult to compare the research results of ROP epidemiology since not only statistical data given are varied in different studies but also inclusion criteria, diagnostics methods, ethnicity, risk factors, newborn survival rates, and health care standards. Considering the experience of the countries where ROP has been taken up for over 10 years, 200-250 cases of ROP have been expected every year in Ukraine [2]. However, today, statistical data on this issue in Ukraine is very limited. The purpose. To study the incidence of retinopathy of prematurity in Odessa oblast in children whose birth weights were < 2500 g. Material and Methods This was a retrospective study of 2682 premature babies who were examined in the Filatov Institute from 2009 to 2016. All children were in a group at high risk of ROP development. The inclusion criteria for the high risk group were birth weight <2500 g and gestational age at birth < 35 weeks. Screening of the newborns was carried out on the basis of Departments of Pathology of Premature Infants, Regional Pediatric Clinical Hospital, and Reznik City Pediatric Clinical Hospital. The initial examination was performed at four to six weeks after children’s birth. The examination included ophthalmoscopy under medical mydriasis using a binocular forehead ophthalmoscope with aspheric lenses of 20 and 30 diopters and using an eyelid speculum and a scleral depressor for rotating the eyeball. Follow-up ophthalmoscopy was repeated every two weeks till retinal vasculogenesis was complete with no signs of the disease or once a week when stage I-II ROP was formed. The examination findings were registered according to recommendations of the International Committee for Retinopathy of Prematurity and following the International Classification of Retinopathy of Prematurity (ICROP) [6, 10, 11]. In case of the disease’s progression into prethreshold stage ROP of the first type (any stage ROP in Zone I with plus disease; stage 3 ROP in Zone I with/without plus disease; stage 2 or 3 in Zone II with plus disease) or into threshold stage ROP (stage 3 ROP in Zone I or II with five contiguous or eight cumulative clock hours with plus disease), laser coagulation of the avascular zones of the retina was performed within 72 hours following after the disease had been diagnosed. In case of aggressive posterior ROP (AP-ROP), laser coagulation was performed within 48 hours. Analysis was performed using biostatistical methods. A frequency was estimated using a 95% confidence interval (95% CI). Elements of a meta-analysis were used to integrate the study findings [1]; a Cohran's Q criterion was used to test homogeneity of the data while I2% was calculated to assess the level of heterogeneity; herewith, to integrate the data we used a fixed effects model for homogeneous findings and a random effects model for heterogeneous findings. When analyzing the dynamics of changes in 2009 to 2016 outcomes we used the Chi-squared test for ordered categories [1]. The the significance level was chosen in all cases as P=0.05. Analysis was processed using softwares MedCalc v.16.8.4 (MedCalc SoftWare bvba, 1993-2016) and MedStat (Lyakh Yu.E., Gurianov V.G. 2004 – 2011). Results and Discussion 202 130 children were born in Odessa Oblast (region) within the period of 2009 to 2015. Of them, 11 291 children weighted less than 2500 g.
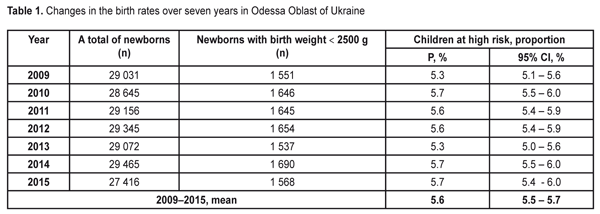
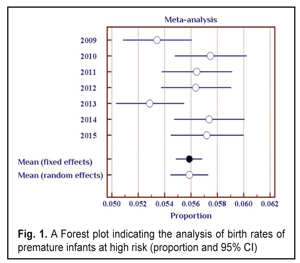
The data in Table 1 demonstrate stable values of birth rates of premature babies at high risk in Odessa Oblast within the period pointed. Thus, during seven years of the follow-up, a ratio of children with birth weight < 2500 g was within 5.3-5.7%. To average the data we used the elements of the meta-analysis (Fig. 1); the heterogeneity test revealed no significant difference in the data (I2=50%, p=0.06).
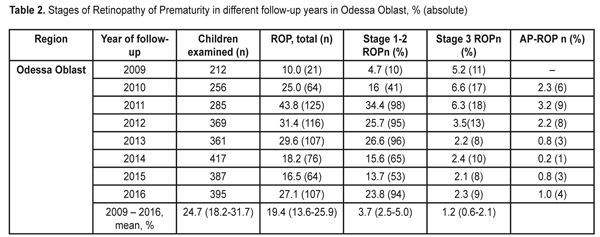
Thus, the values of the birth rates of the premature infants at high risk did not differ within 2009-2015; the fixed effects model was used to average the findings. The mean value of the high risk premature infant birth rates was 5.6% (95% CI: 5.5% – 5.7%). During the follow-up period we examined 2 682 infants. Herewith, the number of the infant examined grew every year and increased almost twice in 2016 as compared to 2009 (Table 2). This tendency can be explained by more effective collaboration with neonatal services of the city and region, an optimized approach to systemic screening of the premature infants, increased awareness of ROP by parents and regional out-patient clinics’ pediatricians.
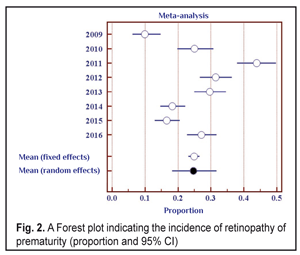
Data in Table 2 shows that as the number of the children examined increased throughout the follow-up period, the number of revealed cases of ROP grew, and the incidence of the disease ranged from 10.0% in 2009 to 43.8% in 2011. To determine the frequency of the ROP cases revealed, we also used the elements of the meta-analysis (Fig. 2). The heterogeneity test revealed a significant difference in the data (I2=94.2%, p<0.001). Thus, the ROP incidence in the period between 2009 and 2011 differed significantly; to average the findings, the random effects model was used. The mean value of the ROP incidence in the period of 2009-2016 was 24.7% (95% CI: 18.2% – 31.7%).
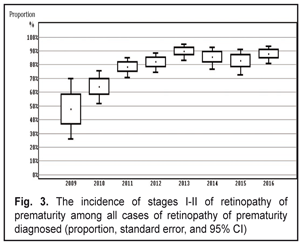
When analyzing the incidence of ROP stages 1 and 2 and testing the heterogeneity, we also revealed a significant difference (the heterogeneity level: I2=94.0%, p<0.001). The incidence of stage 1-2 ROP differed significantly within 2009-2016 with the findings averaged by the random effects model; the mean value of the stage 1-2 ROP incidence was 19.4 % (95% CI: 13.6% – 25.9%). In cases when laser treatment was required (stage 3 AP-ROP), the heterogeneity test revealed a significant difference in the data within the period of 2009-2016 (the heterogeneity level: I2=77.8%, p<0.001). The frequency of such cases ranged within 2009-2016 and its mean value equaled 4.9 % (95% CI: 3.3% – 6.8%) with the findings averaged by the random effects model. Herewith, stage 3 ROP was revealed in 3.7 % of cases (95% C: 2.5%–5.0%). AP-ROP was in 1.2% of cases (95% CI: 0.6%–2.1%). We analyzed the dynamics of changes in the stage 1-2 ROP incidence among all ROP cases revealed within 2009-2016 (Fig. 3).
The presence of the positive trend was revealed (p<0.001 according to the Chi-squared test for the presence of the trend for the ordered categories). Thus, after the systematic screening had been implemented, the incidence of early stage ROP increased (p<0.001) and of all ROP cases revealed, the stage 1 and 2 ROP incidence was 47.6% in 2009 vs. 87.9% in 2016. The incidence of ROP in the children examined in Odessa Oblast increased from 10% in 2009 to 27.1% in 2016. The stage 1-2 ROP incidence increased from 4.7 in 2009 to 23.8 % in 2016. The incidence of stage 3 ROP decreased to 2.3% in 2016 as compared to 5.2% in 2009. In 2016, AP-ROP was diagnosed in 1.0 % of cases. Laser treatment was required for 4.9 % of children. According to the protocol of treatment of children with retinopathy of prematurity (No 683 from 21.09.2009, Ministry of Health of Ukraine), all children born at 22-37 weeks must be examined in order to reveal ROP. However, as it can be seen from our study findings, we had no possibility to screen all premature infants with criteria pointed. This was so because they had been already dismissed from the hospital at four to five weeks, i.e. by the moment of the initial examination. This resulted in the fact that it was more difficult to organize a specialized ophthalmic examination of infants with birth weight > 2000 g. The exception was for cases when the infant were staying in hospital due to severe general condition. Less mature infants were hospitalized so their examination was carried out according to the plan. As a result, we examined the infants with birth weight < 2500 g and gestational age at birth < 35. The inclusion criteria for the high risk group are different in different countries and regions. Thus, in China, in a group of infants with birth weight < 2000 g and gestational age at birth < 34, the ROP incidence rates were 15.0%; 9.87 % of the infants required surgical treatment. In India, infants with birth weight < 1700 g and/or gestational age at birth < 32 have been examined. Here, the ROP incidence rates were 16.5%; 6.7 % of the infants required surgical treatment. Scientists in Rio de Janeiro have carried out a large-scale research and found out that ROP incidence in children with birth weight < 2000 g in the region was 16.9 %, of them 3.6 % required treatment. In South Africa, ROP was diagnosed in 16.3% of children; 6.4% of children were revealed severe stage ROP [3, 5, 7, 12, 13, 14, 17]. Taking into account the fact that when forming the group at high risk in the study we expanded the parameters of birth weight to 2500 g and gestational age to 35 weeks as compared to the countries mentioned above, we revealed a greater number of premature children with the disease. Firstly, that was in account of more mature newborns with stages 1 and 2 and a self-regressed disease. However, in our study there were a less number of children requiring treatment, which can be caused by the fact that a less number of children were born with low birth weight and, respectively, with severe ROP development that required treatment. The frequency of the ROP cases and severe ROP courses in our study correlated with findings obtained in other countries with middle- and low-income, however, clear criteria for the group at high risk of ROP development were not determined. The ROP incidence data obtained in our study can partially indicate the state of the ROP issue in Ukraine. In future, it is required to carry out multi-center researches, to use unified inclusion criteria, and to use corresponding methods of ROP diagnostics when performing the epidemiological assessment. Thus, it will be possible to analyze the ROP incidence level, to improve the scientific quality of research as well as to collect and analyze data on this issue. Conclusions Firstly, the incidence of retinopathy of prematurity increased in Odessa Oblast from 10% in 2009 to 27% in 2016. Secondly, the incidence of stage 1-2 ROP increased from 4.7 % in 2009 to 23.8 % in 2016. The incidence of stage 3 ROP decreased to 2.3% in 2016 vs. 5.2% in 2009. In 2016, aggressive posterior ROP was diagnosed in 1.0 % of cases.
Thirdly, the frequency of laser treatment decreased to 3.3% in 2016 compared to 5.2% in 2009. References 1. Petrie A, Sabin C. [Medical Statistics at a Glance]. Translated from English, V.P. Leonov, translator. M.: GEOTAR-Media; 2003. 144 p. Russian. 2.Rykov SA, Barinov YV. [Modern ways of solving the problems of blindness and low vision due to retinopathy of prematurity in Ukraine]. Oftalmologiia. Vostochnaia Evropa; 2012;3(14):12-7. Russian. 3.Anil Narang, Amod Gupta, Vinekar et al. Retinopathy of prematurity in Asian Indian babies weighing greater than 1250 grams at birth: ten year data from a tertiary care center in a developing countryю Indian Journal of Ophthalmology. 2008.Jan; 55(5):331-6. 4.Backhouse O. Causes of decreased vision and blindness in Madagascar. Community Eye Health. 1996;17:14-6. 5.Chao Chen, Yequn Zhou, Wenjing Shi. The incidence of retinopathy of prematurity: a prospective multicenter study in China. Word ROP Congress III. 2012. OR-02. 6.Committee for the Classification of Retinopathy of Prematurity. An International Classification of Retinopathy of Prematurity. Arch Ophthalmol. 1984;102:1130-134. 7.Dordi Austeng, Karin BM K?llen, Uwe W Ewald et al. Incidence of Retinopathy of Prematurity in Infants Born Before 27 Weeks’ Gestation in Sweden. Arch Ophthalmol.2009;127(10):1315-1319. 8.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020 – the right to sight. Bull World Health Organ. 2001:79:227-32. 9.Goggin M, O’Keefe M. Childhood blindness in the Republic of Ireland: a national survey. Br J Ophthalmol. 1991:75:425-9 10.ICROP Committee for Classification of Late Stages ROP. An international classification of retinopathy of prematurity, II: the classification of retinal detachment. Arch Ophthalmol. 1987:105:906-12. 11.International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005:123(7):991-9. 12.Juan Carlos Silva. Epidemiology of ROP in Latin America. Word ROP Congress III. 2012. OR-03. 13.Mahmoud Nassar. Screening for Retinopathy of Prematurity: The first report from Upper Egypt. Word ROP Congress III. 2012. OR-07. 14.Phan MH, Nguyen PN, Reynolds JD. Incidence and severity of Retinopathy of Prematurity in Vietnam, a developing middle-income country. J Pediatr Ophthalmol Strabismus. 2003;40:208-12. 15.Section on Ophthalmology American Academy of Pediatrics; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572-6. 16.Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J AAPOS.1999;3(1): 26-32. 17.Chen Y, Li X. Characteristics of severe retinopathy of prematurity patients in China: a repeat of the first epidemic? Br J Ophthalmol. 2006;Mar 90(3):268 - 71
|