J.ophthalmol.(Ukraine).2017;5:15-20.
|
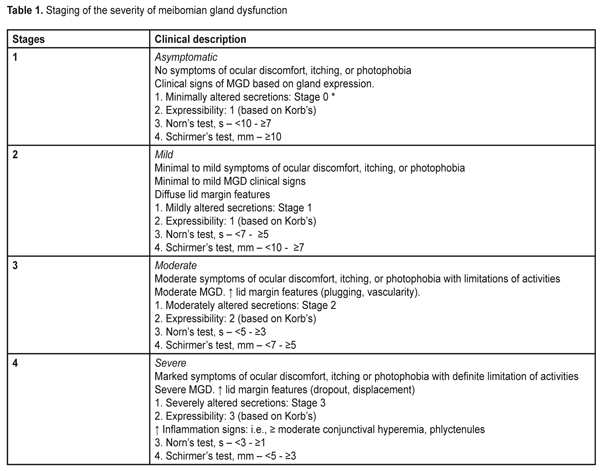
https://doi.org/10.31288/oftalmolzh201751520 Character of meibomian gland dysfunction in patients with diabetic polyneuropathy with severe complications P.A. Bezdetko, L.I. Ivzhenko Kharkiv National Medical University Kharkiv (Ukraine) E-mail: ludok26@yandex.ru Background. Meibomian gland dysfunction (MGD) is a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. Diabetic polyneuropathy can be a cause of MGD. Purpose: To improve the efficacy of diagnosis of MGD in patients with severe complications of diabetic polyneuropathy. Materials and Methods: Standard ophthalmological examination, the Schirmer’s test before and 2 hours after eyelid compression, the Norn’s test, the OPI test, a compression test, an IVAD test, contact meibography with green filter were performed. Severe complications of diabetic polyneuropathy (N3 stage) were recorded in 25 patients (50 eyes). A control group consisted of 97 persons (194 eyes) without diabetes. Results and Discussion: The Schirmer’s test was 2.7 times reduced in patients with N3 stage (4.83 mm) in comparison with that in the control group (12.82) (p<0.001). The Norn’s test was 4.8 times lower in patients with N3-stage (3.45 sec) than in patients without diabetes (9.48 sec) (p<0.001). The OPI test in patients with N3 stage was 1.9 times lower than that in the control group, (p<0.001). The Schirmer’s test in patients with N3 were 1.2 times higher after compression than before it (p<0.05). According to meibography, in patients with N3, loss area of the meibomian glands corresponded to stage 4. Conclusions: The patients with stage N3 had MGD stage 3 (44 %) and 4 (40 %), while the first stage of MGD was not identified in any patient. Key-words: meibomian glands dysfunction, diabetic polyneuropathy, Schirmer’s test, contact meibography, meibomian gland secretions Background World Health Organization defines diabetic polyneuropathy (DPN) as a disease which is characterized with progressive loss of nerve fibers that leads to the loss of sensation and development of foot ulceration [4]. A number of authors have reported that the development of DPN depends on the compensation of carbohydrate metabolism, the duration and type of diabetes mellitus (DM): DPN is diagnosed in 7.5 to 10% of patients with newly diagnosed type 2 DM [5]. According to other authors, the incidence of DPN in type 1 and 2 DM is almost equal and depends not only on the DM duration but on its treatment success [4]. The anterior segment of the eye can also be affected in DM (investigations by Beaver Dam). Therefore, attention should be given to a pathologic condition of the anterior eye, meibomian gland dysfunction (MGD). Meibomian gland dysfunction (MGD) is a chronic, diffuse pathology of the meibomian glands which is commonly characterized by obstruction of the terminal ducts and/or qualitative/quantitative changes in glandular secretion [3]. It may result in tear film alterations, symptoms of eye irritation, clinically expressed inflammation, and a disease of the ocular surface [6]. The meibomian glands are known to have parasympathetic innervations. The muscles of Riolan surround the excretory ducts of the meibomian glands and have effect on secretion [7, 9]. As it’s been mentioned before [4], the nerve fibers and their processes in the central and peripheral nervous system are affected in DPN. It is not unreasonable to assume that alterations occurring in DPN can affect the structure and functioning of the meibomian glands, contributing to MGD development. To the best of our knowledge, there are no such data in the literature. The purpose of the present paper was to increase the efficacy of diagnostics of meibomian gland dysfunction in patients with severe complications of diabetic polyneuropathy. Material and Methods A stage of severe complications of diabetic neuropathy (N3 stage) was registered in twenty-five patients (50 eyes). The diagnosis was made by a neurologist and based on the occurrence of complications (diabetic foot, ulceration, deformation, amputation) and incapacitation. An acute decrease or loss of all sensation types was noted, which resulted in foot traumatism and development of trophic ulcers and purulent-necrotic complications; also, there was reflex and motor insufficiency, sweat abnormality, cold feet, atrophy of soft tissues of the foot, deformation of the joints, a blue skin, claw fingers, plantar callosity. Trophic ulcers were small (1-2 cm in diameter), almost painless formations having a form of deep defects grounded on the tendon, joint surface, and bones. They mostly occurred on the medial surface of the great toe, heel, and foot dorsum and foot balls. Women and men comprised 48% (12 persons) and 52 % (13 persons), respectively. The patients aged from 55 to 77 years old. The DM duration in most patients (68%, 17 persons) was >10-15 years; that was 5-10 years and > 15 years in 12% (3 patients) and 20% (5 patients), respectively. DM was decompensated in most patients (84%, 21 patients); subcompensation was registered in 16 % (4 patients) of cases. All stage N3 DPN patients had a severe run of diabetes. Diabetic retinopathy (DR) was noted in all eyes of the patients with stage N3 DPN: non-proliferative in 10% of cases (5 eyes), pre-proliferative in 14% (7 eyes), and proliferative in 76% (38 eyes). To obtain more objective information on the prevalence and character of meibomian gland dysfunction in diabetic patients, we carried out similar examinations in 97 persons (194 eyes), 51 women and 46 men, without diabetes mellitus, who comprised Control group. They were patients having applied for preventive check up. The group included patients over 40 years, with no history of glaucoma, and whose intraocular pressure did not exceed 24 mmHg. Only emmetropic (or mild hyperopic or mild myopic) eyes were included in the study. The patients aged from 46 to 65 y/o.. A concomitant somatic disease was noted in 20.4% (19 persons) of Control group, in particular: general atherosclerosis and hypertensive disease, 12.9% (12 persons); ischemic heart disease, 7.5% (7 persons). So, Study and Control groups were comparable with respect to gender, age, and a concomitant somatic disease. Together with standard ophthalmic examination (visual acuity test, biomicroscopy, ophthalmoscopy), we performed the Schirmer’s test before and two hours after eyelid compression, the Norn’s test, a compression test to assess the expressibility and the quality of meibum, contact meibography using the green light, and the Interval Visual Acuity Decay and Ocular Protection Index tests. Tear production and the state of the tear film was assessed using the Schirmer’s test and the Norn’s test. To specify the data obtained, the Schirmer’s test was also performed two hours after eyelid compression (at the time of MG secretion renewal) [2] with a glass spatula. After this procedure, it is possible to mark the changes which will evidence the presence of dry eye syndrome (DES) or MGD. In DES, the Schirmer’s test values do not differ significantly after eyelid massage (Ukraine’s utility patent No114141 “A method of dry eye syndrome diagnostics” from 27.02.2017). To assess the meibum expressibility and quality the compression test was performed using a slit lamp, PS-615, Topcon, by compression of the eyelid on a glass spatula with a finger. This examination covered the central third of the eyelid (about five meibomian glands) and expressibility of the meibomian glands was assessed after small compression. Depending on the number of glands expressible, we determined the degree of meibum expressibility according to a scale as follows: Stage 0 or 0 scores – all glands expressible Stage 1 or 1 scores – three to four glands expressible Stage 2 or 2 scores – one to two glands expressible Stage 3 or 3 scores – no glands expressible This test makes it possible to determine the minimal number of the glands that is necessary to provide an adequate tear film lipid layer. If the most of the glands function normally, it is a low probability of dry eye syndrome development; but the probability is high if there are less than four glands with normal functioning [1, 8]. The quality of expressed meibum was also assessed. The meibum quality of eight glands in the central part of the eyelid was assessed and the mean value was determined. The meibum quality was assessed according to alterations and scored according to a scale of 0 to 3: Stage 0 or 0 scores (minimal alterations) – clear Stage 1 or 1 scores (mild alterations) – cloudy Stage 2 or 2 scores (moderate alterations) – cloudy with debris (granular) Stage 3 or 3 scores (severe alterations) – thick, like toothpaste [1, 8] Ocular Protection Index (OPI) was assessed as a ratio of the Norn’s test values and the mean blink interval. Time was kept with a stopwatch and the test was performed 2-3 times and the average of the data obtained was accepted as a result. If the OPI score is 1, then each blink matches tear film break-up time. So, those whose OPI score is <1 have sufficient problems with the tear [11]. Interval Visual Acuity Decay (IVAD) was performed using Landolt C optotypes. First, visual acuity was assessed in patients; then they were asked not to blink as long as possible after which we measured visual acuity before blink and the time between blinks. The method makes it possible to reveal a decrease in visual functions associated with tear film instability [10]. Contact meibography with the green light (Ukraine’s utility patent No 112809 from 27.02.2017) was performed using a diaphanoscope, Heine HK - 150-2 multi; illumination was changed at the fiber input, in the TOPCON-PS61E slit lamp. A Delta Optical PRO camera, 1.3 megapixels, set instead of the slit lamp lens, was used to take photofixation. A diaphanoscope probe contacted the everted eyelid which was illuminated by the green color, and the number and state of the meibomian glands were studied. The findings were assessed according to a scale [12] enabling to evaluate the loss of glands within the area studied and to determine the severity of MG damage, where: Stage 0: area of loss ? 0% Stage 1: area of loss ? 25% Stage 2: area of loss 26%–50% Stage 3: area of loss 51%–75% [13]. To determine the MGD stage and treatment we used data given in Table 1 [14].
Statistical data processing was performed using variance analysis with a Microsoft Excel 2010 software program and a Statplus v5 program. To analyze the data obtained, the significance of the difference was assessed using Student-t criterion. We calculated an arithmetic mean value (M), standard deviation (?), standard error of the mean (mM), coefficient of variation (С?), variance of difference between the means (t), and significant difference (p). Bonferroni correction was considered when comparing the values of more than two groups. For repeated measures analysis we used paired Student's t-test. Spearman rank correlation analysis (r) was also performed. The accepted level of statistical significance was p < 0.05. Results and Discussion Visual acuity in the patients with severe diabetic neuropathy complications was 2.4 times lower than that in the Control group patients, 0.40±0.01 vs. 0.94±0.03, respectively (р<0.001) (Fig. 1).
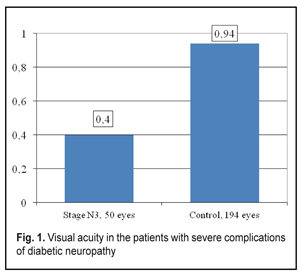
General tear production in patients with severe DPN complications was sharply reduced (Fig. 2).
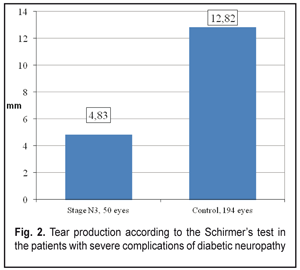
Thus, the stage N3 patients were recorded a decrease in summed tear production which was 2.7 times lower than that in the Control group patients, 4.83±0.31 mm vs. 12.82±0.18 mm, respectively. The Schirmer’s test values in the stage N3 patients were 3.1 times decreased as compared to the norm (15 mm) (р <0.001). Tear film break up time in the stage N3 patients was below normal and 2.7 times lower than that in Control group, 3.45 ± 0.27 s vs. 9.48 ± 0.08 s, respectively, (р <0.001), which can be seen in Figure 3.
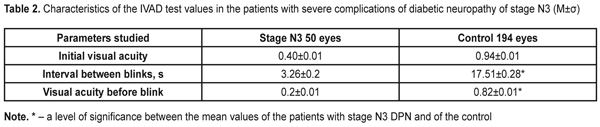
To evaluate the stability of the tear film, each patient was performed the IVAD test, the findings of which are given in the Table 2.
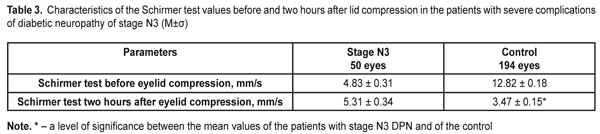
Time between blinks was by 14.25±0.08 s higher in the Control group patients than in the DPN stage N3 patients. Visual acuity before blink was 4.1 times higher in the Control group patients than in the DPN stage N3 patients (р<0.001). The OPI test was used to determine tear film defects. The diabetic patients with stage N3 DPN had the OPI findings below normal and lower than those of the Control group patients, 0.51±0.02 vs. 0.97±0.02, respectively. So, this parameter in the Control group patients 1.9 times exceeded that in stage N3 patients (р<0.001). To specify the Schirmer’s test and to assess the functioning of the lipid layer, we performed the Schirmer’s test before and two hours after compression of the eyelids (Table 3).
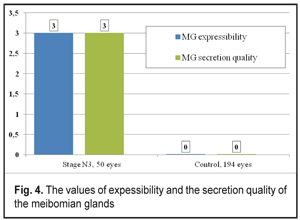
The findings of the Schirmer’s test significantly increased after lid compression in the Control group patients (13.47±0.15 mm). In the DPN stage N3 patients, the Schirmer’s test findings were 1.1 times higher after compression that before it (р<0.05). Meibography in the DPN stage N3 patients determined stage 4 MGD according to the number and area of expressive MGs. In patients with severe diabetic complications of DPN, a significant decrease in gland expressibility, compared to the Control group, was noted (р<0.001). So, the index characterizing the number of expressing glands corresponded to stage 3 MGD in the DPN stage N3 group and significantly differed from that in the Control group (stage 0 MGD) (Figure 4). In DPN stage N3 patients there were significant changes in the quality of secretion, which corresponded to stage 3 MGD. No changes in the quality of sectretion were normally noted in the Control group (stage 0) (Figure 4).
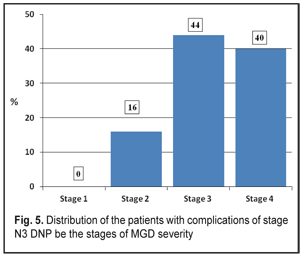
According to data obtained, most of the DNP stage N3 patients had stages 3 (11 persons, 44 %) and 4 (10 persons, 40 %) of MGD while stage 1 of MGD was revealed in none of the patients (Figure 5).
Clinical changes in the structure and state of the meibomian glands were noted in the patients with severe complications of diabetic polyneuropathy compared to the patients of the Control group: 1. summed tear production in the patients with stage N3 diabetic neuropathy (4.83 mm) was decreased 2.7 times as compared with that in the Control Group (12.82 mm) (р<0.001) and 3.1 times as compared with the norm; 2. the Norn’s test in the patients with stage N3 diabetic neuropathy (3.45 s) was 4.8 times lower than that in the patients without diabetes mellitus (9.48 s) (р<0.001); 3. the IVAD values of the patients with stage N3 diabetic neuropathy differed from those of non-diabetic patients. The time before blinks was by 14.25±0.08 s higher in the Control group as compared to the DNP stage N3 patients. Visual acuity before blink was 4.1 times higher in the Control group as compared to the DNP stage N3 patients (р<0.001). 4. the OPI test values were 1.9 times lower in the DPN stage N3 patients that in the Control group patients (р<0.001); 5. the Schirmer test values in the DPN stage N3 patients were 1.2 times higher after compression than before it (р<0,05); 6. Meibography showed that the loss area of the glands corresponded to MGD stage 4 in the DPN stage N3 patients. Expressibility in the group corresponded, at average, to MGD stage 3 and significantly differed from that in the Control group (stage 0); 7. the DPN stage N3 patients had markedly altered secretion which corresponded to MGD stage 3. No alterations in the quality of secretion were normally noted in the Control group (stage 0); 8. most of the DPN stage N3 patients had stages 3 and 4 of MGD, 11 persons (44 %) and 10 persons (40 %), respectively, while MGD stage 1 was revealed in none of the patients (Figure 5). References
2. Blackie CA, Korb DR. Recovery time of an optimally secreting meibomian gland. Cornea. 2009;28:293–297. 3. Bron AJ, Benjamin L, Snibson G. Meibomian gland disease. Classification and grading of lid changes. Eye. 1991;5:395-411. 4. Boulton JM, Malik RA, Arezzo JC. Diabetic Somatic Neuropathies. Diabetes Care. 2004;27(6):1458-86. 5. Feldman EL. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J. Clinic. Investigation. 2003;111:431-3. 6. Foulks G, Bron A. A clinical description of meibomian gland dysfunction. Ocul Surf. 2003;107-26. 7. Jester JV, Nicolaides N, Smith RE. Meibomian gland studies: histologic and ultrastructural investigations. Invest Ophthalmol Vis Sci. 1981;20:537-47. 8. Kelly K Nichols. The International Workshop on Meibomian Gland Dysfunction: Executive Summary. IOVS, Special Issue. 2011;52 (4). 9. Kozak I, Bron AJ, Kucharova K. Morphologic and volumetric studies of the meibomian glands in elderly human eyelids. Corne. 2007;26:610-4. 10. Nelson JD, Shimazaki J, Benitez-del-Castillo JM et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52:1930–7. 11. Ousler GW 3rd1, Hagberg KW, Schindelar M, Welch D, Abelson M. The Ocular Protection Index. Cornea. 2008;Jun; 27(5):509-13. 12. Pflugfelder SC, Tseng S, Sanabria O et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38-56. 13. Pult H Meiboscale. Dr Heiko Pult. Optometry & Vision Research. 2015; Accessed February 10. 14. Tomlinson A, Bron AJ, Korb DR et al. The international workshop on meibomian gland dysfunction: report of the clinical trials subcommittee. Invest Ophthalmol Vis Sci. 2011;52:2006-49.
|