J.ophthalmol.(Ukraine).2017;5:50-55.
|
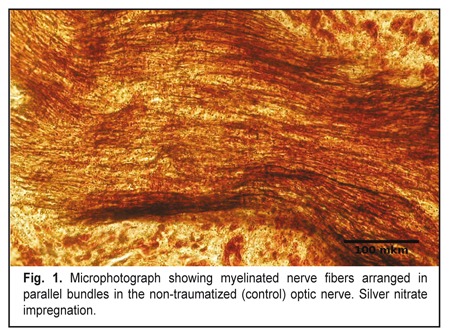
https://doi.org/10.31288/oftalmolzh201755055 Morphological changes in the optic nerve after experimental injury followed by treatment with stem cells I.V. Chepurnyi, A.V. Kopchak, A.V. Korsak, V.V. Likhodievskyi, O.I. Kovalchuk, S.S. Olefir, A.O. Zabila, I.B. Chaikovskyi Bogomolets National Medical University Kyiv (Ukraine) E-mail: yuchaika@i.ua Background: Ocular trauma and its sequelae substantially affect quality of life. Effective methods to promote regeneration of damaged optic nerve (ON) are still to be developed. Purpose: To investigate structural changes in the rat ON after experimental ON injury followed by treatment with stem cells. Materials and Methods: After being subjected to experimental injury, 40 rats were divided into two equal groups depending on whether they did (Group II) or did not receive (Group I) stem cells derived from the bulge of hair follicles of syngeneic animals. Histological and neurohistological techniques were used to investigate structural changes in the traumatized rat ON. Results: Experimental injury to the ON resulted in its degeneration. Stem cell grafting at the site of injury initiated improved ON regeneration, which was evidenced by (1) newly formed nerve fibers and glial columns mostly of oligodendrocytes, (2) reduced glial scar volume due to reduction in the number of astrocytes, and (3) rapid elimination of myelin debris. Conclusion: Injection of neural crest stem cells derived from the bulge of hair follicles of syngeneic animals improves the optic nerve restoration after injury. Key-words: optic nerve, trauma, regeneration, stem cells Introduction Trauma has always received much attention in clinical practice. Injuries to the organs of the nervous system have been especially difficult to treat due to their potential for regeneration [1, 2]. When severe, ocular trauma and its sequelae substantially affect quality of life [3, 4]. Although numerous relevant studies have been conducted, and the pathogenesis of optic nerve damage has been thoroughly investigated, effective methods to promote regeneration of damaged optic nerve are still to be developed [5-11]. Treatment strategies capable of effecting major phases of the regenerative process should be developed. The use of stem cells for this purpose has been found promising [12]. Due to their unique capabilities, they have the potential for achieving the required effect [13, 14]. They are capable, in particular, of differentiating into functionally active cells of the body, and can produce biologically active substances including various growth factors [13, 15- 16]. The purpose of this study was to investigate structural changes in the rat optic nerve after experimental injury followed by treatment with stem cells. Materials and Methods The study protocol was approved by the Bioethics Committee of the Bohomolets National Medical University. An experimental model of injury to the orbital region of the skull and of the optic nerve in the rat was developed. Forty Wistar rats (weight, 180-220 g) maintained under daylight were divided into two equal experimental groups. With the animal under thiopental sodium anesthesia (50 mg/kg, intraperitoneally), a 2-mm circular cutter was used for creating a marginal bone defect to a depth of 1 mm in the right cheekbone arch without making a discontinuity in the arch. In addition, the optic nerve and oculomotor muscles were separated and, at the middle third of the intraorbital portion of the optic nerve, clamped with forceps for 30 seconds to make damage to orbital soft tissue mass. Thereafter, in rats of Group I, the bone defect was filled with prefabricated fibrin gel. In rats of Group II, the site of injury to the orbital soft tissue mass received postnatal multipotent stem cells, epidermal neural crest stem cells (EPI-NCSCs) derived from the bulge of hair follicles of syngeneic animals [17-22]. In addition, the bone defect was filled with prefabricated fibrin gel. The surgical wound was sutured in layers. At weeks 3 and 6 after experimental injury, the optical nerve (intraocular portion, intraorbital portion, intracanalicular portion and intracranial portion going up to the optic chiasma) was taken for study and compared with that of the normal (control) contralateral orbit. Animals were euthanized with an overdose of thiopental sodium prior to taking the material for study. The material was ?xed in 10% neutral phosphate buffered formalin. Hematoxylin and eosin staining, silver nitrate impregnation, and Spielmeyer staining were used to investigate the structure of the optic nerve. Optic nerves were washed in phosphate buffer solution. Sections of the optic nerve were cut on a freezing microtome and impregnated by the silver nitrate method (“rapid method for impregnation of the components of the peripheral nervous system with silver nitrate”) of Kolomiitsev, Chaikovsky and Tereschenko [23]. In addition, the material was stained for the demonstration of myelin sheaths by the Spielmeyer method. After conventional histological processing, the optic nerve was embedded in paraffin, and longitudinal and transverse sections were cut serially and stained with hematoxylin and eosin. Photographs were taken with a digital camera (C-4040 zoom; Olympus, Tokyo, Japan) attached to a light microscope (Olympus model BX51). ImageJ 1.51a (NIH, Bethesda, MD; http://rsbweb.nih.gov/ij) was used for the analysis of digitized images. Results In the soft tissue content of the unaffected (control) orbit in animals of either group, no changes in oculomotor muscles, fat tissue and optic nerve were observed. The intraorbital, intracanalicular and intracranial portions of the optic nerve consisted of numerous myelinated nerve fibers made of retinal ganglion cell axons (Fig. 1) and arranged in parallel bundles. These fibers extended along the trunk of the nerve, with the fiber bundles subdivided by narrow glial columns. The columns contained a moderate number of oligodendrocytes and astrocytes arranged in two to three rows. The structure of the intraocular portion was somewhat different from those of the above portions, and involved the superficial nerve fiber layer, prelaminar region, lamina cribrosa region, and retrolaminar region. In the prelaminar region, glial cell axon bundles were unmyelinated and are surrounded by astrocytes and capillary-containing connective tissue layers. In the lamina cribrosa region, numerous unmyelinated nerve fibers were seen passing through lamina cribrosa that was composed of connective tissue and had multiple pores that were aligned to allow the passage of ganglion cell processes. The retrolaminar region of the optic nerve was composed of myelinated nerve fiber bundles subdivided by columns of astrocytes, oligodendrocytes and microglia. In addition, it was ensheathed in dura, pia, and arachnoid mater and its structure was similar to those of the intraorbital, intracanalicular and intracranial portions of the optic nerve. The above structure of the rat optic nerve was similar to the structure of the human optic nerve.[11]
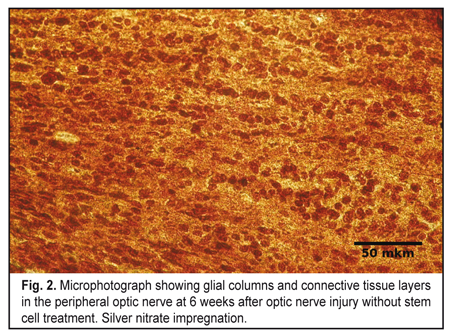
In both experimental groups, at 3 weeks after traumatic injury to the orbital area of the skull, various degrees of destruction of orbital soft tissue mass were observed. At 3 weeks after traumatic injury to the middle third of the intraorbital portion of the optic nerve, the damaged optic nerve in Group 1, as opposed to the control optic nerve, showed such signs of degeneration of the nerve portions as loss of parallel orientation of nerve fibers and of glial cell columns. Parallel orientation of astrocyte columns was observed only in the intraocular portion of, in the lamina cribrosa region of the affected rat optic nerve. Microscopy showed destruction of most of nerve fibers in the central (or retinal) optic nerve extending from the site of injury to the retina and involving the intraocular portion and one-third of the intraorbital portion. In addition, in the intraorbital portion of the central optic nerve, the cells were arranged chaotically among a moderate number of islands of detritus. In this portion, detritus was formed from destroyed myelinated nerve fibers. The number of cells was moderately increased compared to control, with astrocytes being the most abundant, followed by microglia and oligodendrocytes. At the site of optic nerve injury (at the middle third of the intraorbital portion), microscopy revealed chaotically arranged cells and myelin debris separated by moderate amounts of connective tissue. At that site, astrocytes and fibroblasts were the most common, whereas oligodendrocytes and microglia were less common. Myelin debris was found in a moderate number of clumps. At some connective tissue sites, islands of fibrosis appeared as avascular areas. No nerve filaments, increased number of cells and detritus were seen in the peripheral optic nerve going from the site of injury into the optic chiasma and involving one-third of the intraorbital portion and intracranial portion. Compared to the central optic nerve, the islands of myelin debris in the peripheral optic nerve were less numerous and smaller in size, although the detritus area was also substantial compared to the area of the optic nerve. In addition, cells were arranged in a more orderly pattern, with clear intercellular connective tissue layers. At 6 weeks after experimental injury to the orbital area of the rat skull, the damaged optic nerve in Group 1 showed signs of increased degeneration and faint signs of regeneration. Destructive changes were seen in the central part of the optic nerve, with some sites of this part having no nerve filaments, but numerous cells and detritus. In this area, only solitary newly formed axons were seen, which extended only within the intraoculal portion or were limited by that portion and the initial part of the intraorbital portion. In addition, the amount of detritus formed from destroyed myelinated nerve fibers and fragmented axial cylinders increased compared to the previous time-point. The number of cells in the central optic nerve also increased compared to the previous time-point, and was higher than in control. Cells were mostly astrocytes and mictoglia, while oligodendrocytes were less numerous. In the lamina cribrosa region of the intraocular portion, astrocyte columns were oriented in parallel to one another, this arrangement being the same as at the previous time point. As at the previous time-point, (1) in the lamina cribrosa region of the intraocular portion, astrocyte columns were oriented in parallel to one another, and (2) at the site of optic nerve injury, microscopy revealed irregularly arranged cells (astrocytes prevailed, and fibroblasts, oligodendrocytes and mictoglia were also present) and myelin debris separated by connective tissue. Moderate amount of myelin debris appeared as small clamps. The number of islands of fibrosis was increased compared to the previous time-point. At 6 weeks, no nerve fibers, in addition to numerous cells, myelin debris and connective tissue layers were seen in the peripheral optic nerve. Compared to the previous time-point, connective tissue layers separating the relatively regularly arranged rows of abundant cells and clumps of myelin debris were thicker (Fig. 2).
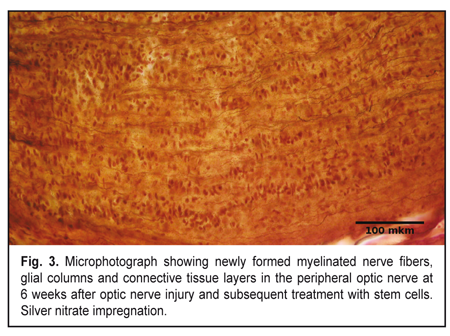
At 3 weeks, signs of optic nerve degeneration were observed not only in Group1, but also in Group 2 (animals receiving post-traumatic stem-cell therapy). However, in Group 2, in contrast to Group 1, the signs of initial regeneration were already present at this time point. The central part of the traumatized optic nerve in Group 2 demonstrated loss of parallel orientation of numerous nerve fibers and presence of parallel orientation of numerous glial cell columns. Among these columns of astrocytes and oligodendrocytes, solitary newly formed axons and myelin debris were seen. In addition, the number of oligodendrocytes was higher than that in animals of Group 1. At the site of optic nerve injury, microscopy revealed chaotically arranged cells and myelin debris separated by connective tissue as in Group 1 at 3 weeks. There were astrocytes among these cells, although their number was lower than in Group 1 at this time point. Fibroblasts, oligodendrocytes and microglia were also present. Myelin debris was found in small-sized clumps. Islands of fibrosis were practically not found in the connective tissue. In Group 2, in the peripheral optic nerve, some regenerating nerve filaments appeared at the site of injury, while newly formed axons were seen only at the initial portion of the peripheral optic nerve. Increased numbers of cells and detritus were also seen. In contrast to Group 1, at this time point, detritus in the peripheral optic nerve contained less myelin debris, and glial columns composed mostly of oligodendrocytes began to appear. Longitudinal layers of connective tissue were seen. At 6 weeks, in Group 2, polymorphic histological picture was seen in the optic nerve. However, in contrast to Group 1, signs of regeneration were more apparent than signs of degeneration. At this time point, in Group 2, the central part of the traumatized optic nerve demonstrated glial columns composed mostly of oligodendrocytes mixed with less number of astrocytes, and newly formed nerve fibers in between the glial cells; however, the number of these fibers was substantially less than in control. In group 2, at the site of optic nerve injury, microscopy revealed chaotically arranged cells and myelin debris separated by connective tissue, as in Group 1 at this time point. However, at this time point, in this group, newly formed myelinated nerve fibers were clearly evident in between cells, detritus and connective tissue, as opposed to Group 1. Ratios of different cell types were similar to those in the same group at the previous time point, although the amount of myelin debris was reduced compared. The size and number of islands of fibrosis in connective tissue were very low. At 6 weeks, newly formed myelinated nerve fibers going from the site of injury to the optic chiasma were found in the peripheral optic nerve in animals of Group 2. These fibers were seen arranged in parallel bundles (Fig. 3) surrounded by numerous glial columns that were formed mostly by oligodendrocytes. At this time point, in Group 2, the detritus to connective tissue ratio was shifted to the right, and wide longitudinal connective tissue layers were rich with microcirculation vessels, as opposed to Group 1.
Discussion Therefore, we developed a model of injury to the orbital area of the scull which demonstrated destructive changes in orbital soft tissue mass and severe degeneration of the optic nerve; this is in accord with the data reported in the literature [24-27]. We would like to mention the following among the factors that have been reported to hamper the optic nerve regeneration process. The lack of axonal regeneration in the injured optic nerve of adult mammals are mainly attributable to 1) delayed axotomy-induced apoptotic cell death of RGCs, 2) insuf?cient intrinsic ability of mature RGCs to regrow axons, 3) growth-inhibitory myelin in the optic nerve, and 4) formation of an inhibitory glial scar at the injury site [5]. In addition, during regeneration of injured nerve fibers, problems arise with regard to axonal regrowth and reconnection with the brain [9, 10]. Postnatal multipotent stem cells (epidermal neural crest stem cells (EPI-NCSCs) derived from the bulge of hair follicles of syngeneic animals) which we used to promote the optic nerve regeneration, have intrinsic capabilities that can be used to achieve the purpose of the study. It has been demonstrated that, when grafted at the site of optic nerve injury, EPI-NCSC cells survived for up to 6 months and showed a low potential for migration and a high differentiation capacity induced by transforming growth factor (TGF)-? that is secreted by lymphocytes, microglia and macrophages. In addition, they, as opposed to fetal stem cells derived from neural crest cells, showed no tumorogenic potential [16]. It has been shown also that one-third of grafted stem cells begin expressing RIP, the oligodendrocyte marker, thus showing evidence of their differentiation into immature oligodendrocytes. The rest of grafted stem cells begin expressing bIII-Tubulin, the oligodendrocyte marker, thus showing evidence of their differentiation into neural cells. Glial ?brillary acidic protein (GFAP) is known to mark differentiated astrocytes, and no GFAP expression was found; however, almost all fetal stem cells derived from neural crest cells differentiated into astrocytes at the site of injury [16]. It has been shown that EPI-NCSCs begin expressing VEGF-A and VEGF-В, these two growth factors promoting vascularization at the site of injury [15, 16]. Analysis of the EPI-NCSC gene profile revealed that these cells express nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) that support the viability of neural cells. It has been established also that axons in the white matter of the spinal cord grow towards grafted EPI-NCSCs. [16] The current study established that stem cell grafting at the site of optic nerve injury initiates improved optic nerve regeneration, which was evidenced by (1) newly formed nerve fibers and glial columns mostly of oligodendrocytes, (2) reduction in glial scar volume due to reduction in the number of astrocytes, and (3) rapid elimination of myelin debris. The presence of nerve fibers in the central and peripheral optic nerve regions in Group 2 at week 6 may be attributed to the ability of stem cells to protect vulnerable RGCs and to the ability of EPI-NCSCs to transform into nerve cells. The emergence of increased numbers of regularly arranged parallel columns of oligodendrocytes may be attributed to the ability of stem cells to differentiate towards immature oligodendrocytes. Reduced glial scarring at the site of injury might be facilitated by the reduction in the number of oligodendrocytes and increase in the number of astrocytes. Rapid elimination of myelin debris may be attributed to the improved vascularization of the traumatized nerve due to the ability of stem cells to express some special growth factors. Conclusion
Injection of neural crest stem cells derived from the bulge of hair follicles of syngeneic animals improves the optic nerve restoration after injury to the orbital skull region and orbital soft tissue mass. References 1.Watanabe M. Regeneration of optic nerve fibers of adult mammals. Dev Growth Differ. 2010 Sep;52(7):567-76. 2.Wender M, Adamczewska-Goncerzewicz Z, Goncerzewicz A. Myelin lipids in Wallerian degeneration of the rabbit optic nerve. Exp Pathol (Jena). 1979;17(6):334-9 3.Gall C, Lucklum J, Sabel BA, Franke GH. Vision- and Health-Related Quality of Life in Patients with Visual Field Loss after Postchiasmatic Lesions. Invest Ophthalmol Vis Sci. 2009; 50:2765–76 4.Greenwald B, Kapoor N, Singh A. Visual impairments in the first year after traumatic brain injury. Brain Inj. 2012;26(11):1338-59 5.Fischer D, Leibinger M. Promoting optic nerve regeneration. Prog Retin Eye Res. 2012 Nov;31(6):688-701 6.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med. 2014 Aug;20(8):857-69 7.Maclaren RE. Regeneration and transplantation of the optic nerve: developing a clinical strategy. Br J Ophthalmol. 1998 May;82(5):577-83. 8.Pernet V, Joly S, Dalkara D, et al. Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol Dis. 2013 Mar;51:202-13. 9.Pernet V, Joly S, Jordi N, et al. Misguidance and modulation of axonal regeneration by Stat3 and Rho/ROCK signaling in the transparent optic nerve. Cell Death Dis. 2013 Jul 18;4:e734 10.Pernet V, Schwab M. Lost in the jungle: new hurdles for optic nerve axon regeneration. Trends Neurosci. 2014 Jul;37(7):381-7. 11.Vit VV. [The structure of the human visual system]. Odessa:Astroprint; 2003 Russian 12.Kordium V, Chaikovsky Yu, Irodov D, Drahulian M, et al. Modelling of systemic lesion of organism for development of multitarget cellular and cytokine therapy. Biopolym Cell. 2016;32(5):381-94. 13.Liu B, Hunter D, Smith A, et al. The capacity of neural crest-derived stem cells for ocular repair. Birth Defects Res C Embryo Today. 2014 Sep;102(3): 299-308. 14.Vasyliev R., Rodnichenko A., Shamalo S. et al. Effects of Neural Crest-Derived Multipotent Stem Cells on Regeneration of an Injured Peripheral Nerve in Mice. Neurophysiology. 2015;47(1):80–83 15.Sieber-Blum M, Schnell L, Grim M, et al. Characterization of epidermal neural crest stem cell (EPI-NCSC) grafts in the lesioned spinal cord. Mol Cell Neurosci. 2006 May-Jun;32(1-2):67-81. 16.Sieber-Blum M. Epidermal neural crest stem cells and their use in mouse models of spinal cord injury. Brain Res Bull. 2010 Oct 30;83(5):189-93 17.Biernaskie J, Sparling J, Liu J, et al. Skin-Derived Precursors Generate Myelinating Schwann Cells That Promote Remyelination and Functional Recovery after Contusion Spinal Cord Injury. J Neurosci. 2007 Sep 5;27(36):9545-59 18.Dupin E, Coelho-Aguiar J. Isolation and differentiation properties of neural crest stem cells. Cytometry A. 2013 Jan;83(1):38-47 19.Krejci E, Grim M. Isolation and characterization of neural crest stem cells from adult human hair follicles. Folia Biol (Praha). 2010;56(4):149-57 20.Sieber-Blum M, Grim M, Hu YF, Szeder V. Pluripotent neural crest stem cells in the adult hair follicle // Dev Dyn. 2004 Oct;231(2):258-69. 21.Sieber-Blum M, Grim M. The adult hair follicle: Cradle for pluripotent neural crest stem cells. Birth Defects Res C Embryo Today. 2004 Jun;72(2):162-72. 22.Yang R, Xu X. Isolation and Culture of Neural Crest Stem Cells from Human Hair Follicles. J Vis Exp. 2013 Apr 6;(74) 23.Kolomiitsev A, Chaikovsky Yu, Tereschenko T. [Rapid method for impregnation of the components of the peripheral nervous system with silver nitrate]. Morfologiia (form. Arkh Anat Gistol Embriol). 1981;8:93-6. Russian 24.Morgan-Warren PJ, Berry M, Ahmed Z, et al. Exploiting mTOR Signaling: A Novel Translatable Treatment Strategy for Traumatic Optic Neuropathy? Invest Ophthalmol Vis Sci. 2013 Oct 23;54(10):6903-16 25.Narciso M, Hoko? J, Martinez A. Watery and dark axons in Wallerian degeneration of the opossum's optic nerve: different patterns of cytoskeletal breakdown? An Acad Bras Cienc. 2001 Jun;73(2):231-43. 26.Saggu SK, Chotaliya HP, Blumbergs PC, Casson RJ. Wallerian-like axonal degeneration in the optic nerve after excitotoxic retinal insult: an ultrastructural study. BMC Neurosci. 2010;11:97 27.Yu F, Zhang R. A novel model of optic nerve injury established by microsurgery using the pterional approach in cats. Neurol India. 2011 May-Jun;59(3):355-61
|