J.ophthalmol.(Ukraine).2017;4:51-54.
|
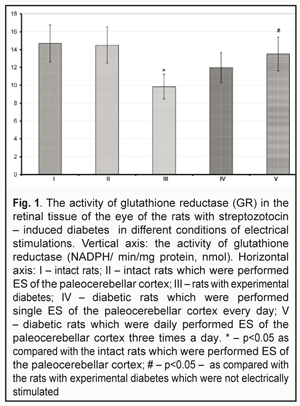
https://doi.org/10.31288/oftalmolzh201745154 To mechanisms of retinopathy development in streptozotocin-induced diabetes against electrical stimulation of brain structures N.V. Kresyun, Prof., Dr. Sc. (Med.), G.A. Son, a Postgraduate Student, L.S. Godlevskii, Prof., Dr. Sc. (Med.) Odessa National Medical University E-mail: talochka.kr@yandex.ua Introduction. Electrical stimulations (ES) of brain structures as well as retinal stimulations have an anti-inflammatory effect and such ES can be used as a method of complex treatment for diabetic retinopathy. The aim of the present paper was to study the activity of glutathione reductase (GR) and of malondialdehyde (MDA) in the retina of the eye, to monitor body mass of rats with streptozotocin (STZ) – induced diabetes as well as to compare changes in these indices in conditions of paleocerebellar electrical stimulations. Material and Methods. Wistar rats were performed ES of paleocerebellar cortex (V-VII lobules) (80-120 mcA, 100 Hz) during four weeks, starting on the 15th day from STZ (55.0 mg/kg, i.p.) administration. At 1.5 months from the moment of the STZ injection, the GR activity and the MDA level were determined in the homogenized retinal tissue and results were expressed as NADPH/min/mg (nM) and nM/mg of protein, respectively. Results. Body weight of the rats with diabetes was increased by 10.7% at the moment of final examination as compared with the baseline indices. In control group of the rats, such increase was 36.2% (P<0.05). That index in diabetes groups treated with ES daily and three times per day was equal to 29.3% and 27.0% (P<0.05), respectively. In the group of the rats which were treated with ES three time per day, the activity of GR in retina increased by 37.0% (P<0.05), аs well as the level of MDA dropped down by 44.5% (P<0.05) pertained to analogous indices in the rats with diabetes. Conclusions: ES of paleocerebellar cortex in the rats with STZ diabetes prevented body weight loss as well as decreasing of GR activity and increasing MDA level in the retinal tissue. Key-words: streptozotocin, diabetes retinopathy, oxidative stress, electrical stimulation, cerebellum. Backgrounds Recently, direct electrical stimulations (ES) of the retina, both noninvasive and with implantation of microchip enabling to generate light-induced ES [9], have been found effective for recovery of the retinal function in experimental retinopathy. And a therapeutic effect of such ES that has been observed is a decrease in the occurrence of oxidative stress and in the production of inflammatory mediators [8]. Such orientation of the indirect effect of ES on the retina should be compared with mechanisms of the therapeutic effect of ESs on the paleocerebellar cortex that has been found effective in experimental diabetic retinopathy in our previous studies [1, 2]. Thus, it has been shown that such ESs postpone the development of retinopathy in streptozotocin-induced diabetic rats, which is evidenced by the fact that components of electroretinogram, immune reactivity and metabolic parameters have been preserved and degenerative processes have been slowed down in the retina [1, 2]. Thus, the purpose of the present paper was to study the activity of glutathione reductase (GR) and of malondialdehyde (MDA) in the retina of the eye, to monitor body mass of rats with streptozotocin (STZ)-induced diabetes as well as to compare changes in these indices in conditions of paleocerebellar electrical stimulations. Materials and Methods The study was performed under long-term experiment conditions in male Wister rats, weighted 170-240 g; the rats were kept in standard conditions at ONMU vivarium. The study followed requirements of the GLP (Good Laboratory Practice) and bioethical committee of ONMU (protocol No 84 from October, 10th, 2008). Under the nembutal anesthesia (40.0 mg per 1 kg, ip), the rats were implanted bipolar nichrome electrodes (the distance between the electrodes was 0.25-0.3 mm) in cerebellar lobules V-VII which were fixed to the cranial surface with the Norakril resin. The animals were followed up beginning from Day 7-10 after performing the surgical intervention. Experimental diabetes mellitus was induced in fasting rats by ip 55.0 mg/kg streptozotocin (STZ) (“Sigma Aldrich.ru”, Moscow) which had been dissolved in a buffer sodium-citrate solution (рН 4.5). At week 1 and 2 after STZ onset, the venous blood obtained from the tail vein was tested and the glucose level was determined; we followed up only those rats in which the glucose level exceeded 300 mg/l [5]. The glucose level test was made at 9 a.m. under the conditions when the experimental animals had free access of food/water during the night time period. During the whole follow-up period, the rats were administered insulin (sc; 0-2 units; five times per week) [5]. The experimental animals were divided into groups as follow: Group 1: control, intact pseudo-operated on rats (11 rats) Group 2: intact rats which were performed ES of the paleocerebellar cortex (12 rats) Group 3: diabetic pseudo-stimulated rats (11 rats) Group 4: diabetic rats which were performed single ES of the paleocerebellar cortex every day (10 rats) Group 5: diabetic rats which were daily performed ES of the paleocerebellar cortex three times a day (10 rats) At Day 14-15 after STZ onset and during the following four weeks, ES of the paleocerebellar cortex was performed using the electrodes implanted. To perform ES, we used a universal electric stimulator (ES2, Ukraine) which generated square-wave pulses with a power of 80-120 µA, with impulse frequency of 100 Hz, and SM duration of 2.5 s. We used single (9.00) and three time (9.00; 14.00; 19.00) modes of ES. On follow-up completion, the rats were euthanized and tissues obtained from the decapitated animals were frozen and preserved in liquid-nitrogen. The isolated retinal tissues were washed with phosphate buffer solution in order to remove blood components and homogenized in 0.1 M phosphate buffer solution (рН 7.0) with a ratio of 1:10 (weight/volume). The homogenized samples were centrifuged for 15 minutes with 13 000 r.p.m. and temperature of + 4°С. MDA was determined spectrophotometrically [4]. Thus, the examined homogenate was incubated in the acid medium at a temperature of 40 °C using thiobarbiturates and the obtained solution, pink in color, was examined using spectrophotometer with a light wavelength of 532 nm. A tetraethoxypropane solution served as standard. The level of MDA was expressed as nmol/mg protein. The Lowry method was used to determine the MDA level [4]. The activity of GR (NADPH/ min/mg protein, nmol) was detected using the Goldberg D.M. method [7]. Data obtained were processed using an ANOVA method and a Newman-Keuls statistical test. Results and Discussion By the end of the experimental follow-up, the weight of the control rats was increased by 36.2% as compared with the initial weight and reached 297+ 18.3 g. The increase in the weight of the rats with ES of the paleocerebellar cortex for the same time period was 29.3% and 27.0% for single and three time daily ES, respectively, which did not differ from that in the control (P>0.05) and exceeded its initial value before STZ onset (P>0.05). At the same time, this index was equal to 10.7% (P<0.05) in the diabetic rats without ES. In all groups studied, the glucose level in the blood was 3.3-4.4 times higher than that of the intact rats (P<0.05). The GR activity in the retinal tissue of the diabetic rats without cerebellar ES was 9.85+0.11 nmol (Fig. 1) and was by 33.0% less as compared with that of the intact rats (P<0.05). This index studied in the STZ-induced diabetic rats with single daily cerebellar ES was by 21.8% higher than that in the diabetic rats without ES (P>0.05) and, at the same time, it remained by 18.4% less than that in the intact rats (P>0.05). Against more frequent ESs (three times a day), the GR activity was increased by 37.0% (P<0.05) compared with the index in the diabetic rats and was by 8.2% less than that in the intact rats (P>0.05). The GR activity in the intact rats with three time daily ESs was by 3.7% less than that of control, the intact rats without ES (P>0.05) (Fig. 1).
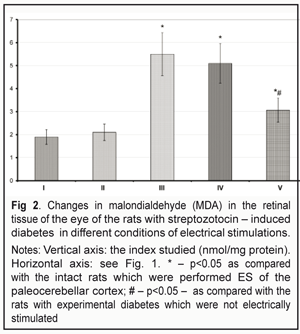
The MDA level in the retinal tissue of the intact rats was 1.90+0.25 nmol/mg protein (P>0.05) (Fig. 2). The same index in the diabetic rats was 2.9 times higher and equaled 5.51+0.72 nmol/mg protein (P<0.05). The MDA level in the diabetic rats with single daily ES was decreased by 7.7 % (P>0.05) as compared to that in the diabetic rats and remained significantly higher (2.63 times) as compared to that in the intact rats of control (P<0.05). The MDA level in the diabetic rats with three time daily ESs decreased to 3.05+ 0.31 nmol/mg protein, which was by 44.5% less than that in the diabetic untreated rats (P<0.05); at the same time, the index remained much higher (1.60 times) compared with that in the intact rats (P<0.05). It should be noted that the MDA level in the retinal tissue of the intact rats with three time daily ES was by 10.5% higher than that in the intact rats (P>0.05) (Fig. 2).
Thus, the data obtained evidenced that the development of experimental streptozotocin-induced diabetes was accompanied by increased peroxide processes in the retinal tissues which were determined as a decrease in the GR activity and the increased level of MDA. Such changes are common for experimental diabetic retinopathy [1, 5]. Besides, the rats were observed significant weight loss associated with diabetes. The disorders pointed were prevented with periodical ESs of the paleocerebellar cortex and the effect of the similar ES in the intact rats was not accompanied by the changes in the indices studied. It should be noted that such a character of the effects of cerebellar ESs, in a great degree, matches the character of the effects of direct retinal ESs. Thus, ES of the retina had a prominent inhibitive effect on the secretion of interleukin (IL)-1? and tumor necrosis factor (TNF)-? in microglia, isolated from Sprague-Dawley rats, and resulted in the increased production of brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF) in M?ller cells [8]. In similar conditions, downregulation of Bax, an apoptotic-associated gene, was observed [11]. Besides, the neuroprotective mechanism is an increase in insulinlike growth factor (IGF-1) production by M?ller cells [9]; ES resulted in elevated fibroblast growth factor-2 in the culture of the retinal M?ller cells [8, 9]. Similar changes as well as differentiated expression of more than 490 genes [6] which is observed in ES conditions, occurs during the first hour, maintains for 35 hours after electrical stimulation and is accompanied by increased production of twenty-five proteins including cell signal proteins, proteins associated with synaptic transmission, metabolic proteins, immunological factors and structural proteins, are considered as a strong anti-inflammatory effect. The pointed profound changes in metabolism of the nerve tissue also resulted in maintenance of the body weight of the diabetic rats, which can be associated with the effect of cerebellar ES on leptin production [3]. Besides, the maintenance of the body weight is an evidence of safety and an important indicator that an ES procedure has no side effects [5]. A possible mechanism of the ES effects on the state of the retina, the character of which corresponds to the effect of the direct ES of the retina, consists in antidromous celereal-tectonic-retinal actions that are provided through corresponding retinal-collicular-perebellar connections [10] and accompanied by the normalized cytokine production, the decreased nitrosative stress and neuroprotection [1, 2]. Since ES of the cerebellar structres is safer than the direct transorbital, transpalpebral, or transcortical ES effect, the further investigations on the optimizing use of the cerebellar ES in ophthalmological practice can be considered reasonable. Conclusions 1. The data obtained evidenced that ES of the paleocerebellar cortex prevented the body weight loss 2. ES of the paleocerebellar cortex prevented the STZ-diabetes-associated decrease in the GR activity as well as the increase in the MDA level in the retinal tissue. 3. ES of the paleocerebellar cortex caused no changes in the indices studied in the retinal tissues of the intact rats.
4. The results obtained give the evidence that ES of the paleocerebellar cortex is safe and can be used in the ophthalmological practice as a component of complex correction for manifestations of retinopathy. References
|