J.ophthalmol.(Ukraine).2017;4:44-50.
|
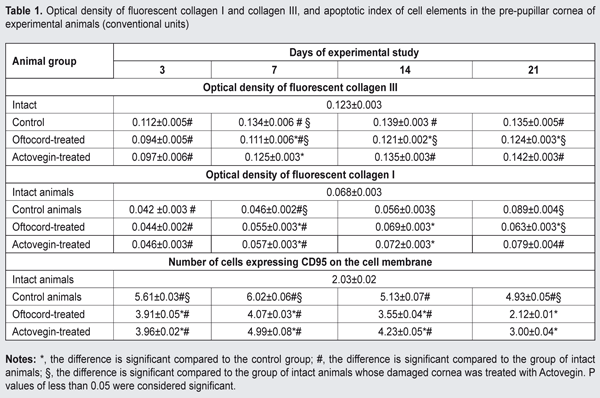
https://doi.org/10.31288/oftalmolzh201744450 Effect of Oftocord gel vs Actovegin on regenerative processes following mechanical trauma to the rabbit cornea N.N. Moisieieva, Cand Sc (Biol), A.K. Gulevsky, Dr Sc (Biol), Prof., O.L. Gorina, Cand Sc (Biol), Z.A. Seidaliieva, Post-grad Student Institute for Problems of Cryobiology and Cryomedicine of the NAS of Ukraine, Kharkiv E-mail: ukrainanataliy@gmail.com Background: Mechanical damage to the cornea is followed by reduced collagen synthesis and imbalance in collagen ratio in the defect area, and by initiation of apoptosis in connective tissue cells, which may result in the transformation of granulation tissue into fibrous tissue. To prevent these alterations, corneal healing processes should be regulated with medications aimed at restoring corneal structure. Purpose: To compare the effect of Oftocord eye gel and Actovegin gel on changes in collagen I and collagen III synthesis and on apoptosis process in the rabbit cornea following mechanical corneal trauma. Materials and Methods: Thirty six Chinchilla rabbits were subjected to a standardized mechanical corneal damage. Thereafter, their corneal defect sites received one of the following treatments for 21 days: placebo gel with no active ingredient (Group 1), Oftocord gel containing a low-molecular weight fraction of human cord blood (HCBF) (Group 2), and Actovegin gel (Group 3). Enucleation for histological and histochemical studies was performed immediately after euthanasia at days 3, 7, 14 and 21. The composition of HCBF was studied and compared with that of Actovegin. Results: Oftocord gel applied to the site of corneal damage prevented the development of apoptosis and improved imbalance in the ratio of newly synthesized collagen I and III in the corneal defect area. The composition of HCBF versus Actovegin was studied to elucidate the mechanism of the effect of Oftocord gel on the metabolism of connective tissue. HCBF and Actovegin substantially differed from each other in composition both qualitatively and quantitatively, with the greatest differences observed in the content of glucuronic acid and of the glycoproteins exhibiting a dominating effect on the metabolism of the connective tissue. Conclusion: Applying Oftocord gel to the site of corneal damage promoted collagen I and III synthesis normalization, and reduction in the percentage of apoptotic cells in the cornea throughout the study. HCBF and Actovegin substantially differed from each other in composition both qualitatively and quantitatively. Key-words: corneal regeneration, apoptosis, collagen I and III, low-molecular weight (below 5 kDa) fraction of human cord blood, Oftocord eye gel Introduction Damage to the cornea is followed by reduced collagen synthesis and imbalance in collagen ratio in the defect area, and by initiation of apoptosis in connective tissue cells, which may result in leucoma [1]. Therefore, at the wound remodeling stage, to prevent the transformation of granulation tissue into fibrous tissue, corneal healing processes should be regulated with medications aimed at restoring corneal structure. Actovegin gel, a hemoderivative produced from suckling calf blood, is such a medication. It has been demonstrated previously that injections of low–molecular weight compounds (below 5 kDa) of cord blood had better wound-healing effect compared to Actovegin injections. In this regard, we hypothesized that a gel developed from a low-molecular weight (below 5 kDa) fraction of human cord blood (Oftocord gel) [2] would be more effective in promoting restoration of corneal structure following mechanical corneal damage. Therefore, the purpose of this work was to compare the effect of Oftocord gel and Actovegin gel on changes in collagen I and collagen III synthesis and on apoptosis process in the cornea following mechanical corneal trauma. Materials and Methods A low-molecular weight (below 5 kDa) fraction of human cord blood (HCBF) was derived by ultrafiltration from human cord blood cryohemolisate [3, 4]. Thereafter, the HCBF was lyophilized and introduced into gel. Actovegin 20% eye gel or 40 mg/mL Actovegin in ampoules (Actovegin®, Nycomed, Linz, Austria) was used as a comparator agent. Thirty six Chinchilla rabbits (weight, 2.2 ± 0.4 kg) were used in the study. Out of them, 27 animals were used in the model of bilateral standardized trephine-induced mechanical corneal defect that has been described previously [5], with the experiments performed after topical anesthesia. Fluorescein staining was used to assess the area of corneal defects. Following replication of the model, these 27 animals were divided into three groups of 9 animals each, and their corneal defect sites received one of the following treatments 4 times a day for 21 days: placebo gel with no active ingredient (Group 1), HCBF-containing Oftocord gel (Group 2), and Actovegin gel (Group 3). To prevent bacterial complications, each gel application in experimental animals was preceded by introduction of antibiotic eye drops (Levofloxacin) to the defect area. Group 4 (9 intact animals) was not subjected to experimental procedures. Enucleation for histological and histochemical studies [6] was performed using a conventional surgical technique after euthanasia at days 3, 7, 14 and 21. Immunofluorescence was used to determine 1) the levels of type I collagen and type III collagen with FITC-labeled monoclonal antibodies (mAB) specific for these types of collagen and 2) the numbers of apoptotic cells expressing CD95 (Novocastra Laboratories Ltd.) in the damaged cornea. FITC-labeled F(ab')2 fragments of rabbit antibodies against mouse immunoglobulin were used as fluorescent labels. The immunofluorescence reaction was studied using the method of Brozman [7]. Fluorescence brightness was registered and antigen content in the tissue was quantified (arbitrary units) using the method of Hubina-Vakulyk [8]. Estradiol, free triiodothyronine, cortisol and progesterone in HCBF and in the comparator agent (Actovegin) were measured with electrochemiluminescence immunoassays (ECLIA) for the in vitro quantitative determination of the hormones in biological fluids on the Elecsys 1070/2010 analyzer (Hoffmann-La Roche, Basel, Switzerland). Levels of ionized calcium, magnesium, phosphorus, zink, glucose, and creatinine were measured with electrochemiluminescence immunoassays on the Elecsys 1070/2010 and Cobas C311 analyzers (Hoffmann-La Roche, Basel, Switzerland). Chondroitin sulfate levels were determined using the method described previously [9]. Glycopeptides levels were determined using the method of Shteinberg and Dotsenko [10]. Uronic acid levels were determined using the method described previously [11]. Total sulfated glycosaminoglycan (GAG) levels were assessed using the method described previously [12]. Low-molecular-weight protein and peptide components of HCBF and Actovegin samples were assessed by gel permeation chromatography [13] with a plastic column (1.6 x 40 cm) packed with a gel medium (TSK-Gel Toyopearl HW-40F, Toyo Soda Manufacturing Co, Ltd, Tokyo, Japan). Sample volume was 0.2 mL, and the dry weight concentrations of Actovegin and HCBF corresponded to 40 mg/mL in normal saline. Phosphate buffered saline (PBS; Na2HPO4/ NaH2PO4 30 mM, NaCl 100 mM; pH 7.4) was used as eluent. The column was calibrated with conventional marker proteins, bovine serum albumin (BSA) (67 kDa), ovalbumin (45 kDa), cytochrome C (12.3 kDa) and blue dextran (2,000 kDa). All animal procedures were performed in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (European Council, ETS No. 123). The animals were housed under standard vivarium conditions. Data were analyzed using "STATGRAPHIC plus for Windows" version 2.1 (StatPoint, Inc., Herndon, VA, USA). Mann-Whitney test was used for comparison of groups. Data are presented as means ± SD [14]. Results and Discussion Immunohistochemistry revealed the presence of the two interstitial collagens, a relatively young collagen type III and mature collagen type I, in the cornea (most commonly, in the stroma) of intact animals, with the levels of the former collagen being higher than the latter (Table 1).
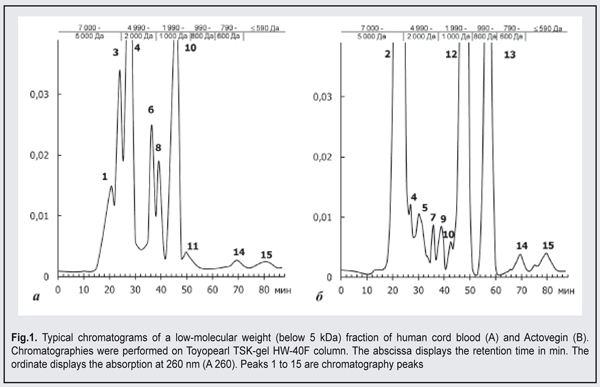
Following mechanical corneal damage, collagen content at the site of corneal damage in control animals became substantially lower than in intact animals. Thus, at day 3, the optical density of collagen I and III staining in the cornea of control animals was 0.112±0.005 conventional units and 0.042±0.02 conventional units, respectively. At the later time points, excessive levels of collagen I and III were revealed in the stroma of the regenerating cornea. Following application of Oftocord gel to the site of corneal damage in animals of Group 2, the content of collagen III at the site was higher than the content of collagen I at all time points, but the changes over time in collagen production were substantially different from those in control animals. In particular, the optical density of collagen III in the damaged cornea was not higher than in the cornea of intact animals, and was statistically significantly lower than in the cornea of control animals throughout the study period. At day 14, the optical density of collagen I and III in the site of corneal damage in Oftocord gel-treated animals was not statistically significantly different from that in the cornea of intact animals. In Actovegin gel-treated animals, the changes over time in collagen III (but not in collagen I) synthesis in the site of corneal damage were not different from those in control animals. Thus, collagen I content in the regenerating cornea was not different from normal values at day 7, but was as high as 0.079±0.004 at day 21, which was significantly (p <0.05) higher than normal values. Therefore, application of Oftocord gel to the site of corneal damage promoted normalized collagen I and III synthesis at the site. Applying Actovegin gel to the site of corneal damage resulted in excessive collagen synthesis, which may be followed by corneal fibrosis and loss of sight. In the next series of experiments, we investigated the effect of HCBF-containing Oftocord gel on the severity of apoptotic changes in the regenerating cornea following mechanical damage to the cornea. We conducted fluorescent microscopy and found low numbers of cells expressing CD95 and exhibiting moderate fluorescence intensity in the corneal limbus and stroma of intact animals, the mean percentage of these cells being as low as 2.03±0.02% (Table 1). At day 3 after mechanical damage to the cornea, the percentage of apoptotic cells in control animals substantially increased (Table 1). In rabbits treated with HCBF-containing gel, the percentage of apoptotic cells at the site of damage at days 3, 7 and 14 was statistically significantly higher (p < 0.05) than in intact animals, but lower than in control animals. In addition, the percentage of cells expressing CD95 at the site of damage at day 21 was not different from normal values. Although in rabbits treated with Actovegin gel, the percentage of apoptotic cells at the site of damage was substantially lower compared to control animals throughout the follow-up period, it was not as low as normal values at day 21. Therefore, we found that applying Oftocord gel to the site of corneal damage prevented the development of apoptosis and imbalance in the ratio of newly synthesized collagen I and III in the defect area, and thus hampered excessive scarring in the damaged cornea [15].We investigated the composition of the main active component of Oftocord gel, a low-molecular weight (below 5 kDa) fraction of human cord blood, versus Actovegin (the dry weight concentrations of Actovegin and HCBF corresponded to 40 mg/mL in normal saline), to elucidate the mechanism of the effect of Oftocord gel on collagen synthesis. Low-molecular-weight protein and peptide components of HCBF and Actovegin samples were assessed by gel permeation chromatography. The comparison of the chromatographic profiles indicated substantial differences in peak positions and quantitative differences in their low-molecular (0.3 to 0.5 kDA) protein and peptide components (Fig. 2). The chromatograms presented demonstrate a substantial difference in peak areas between a low-molecular weight HCBF and Actovegin.
The chromatographic profile of HCBF shows 10 main peaks, including the peaks (peaks 2, 5, 7, 9, 12, and 13) not characteristic of Actovegin. A substantial portion (53.38%) of HCBF components appear in peak 2, with their molecular weight ranging from 5 to 5.2 kDA. This peak was not found in the comparator samples. The main portion (34.98%) of the protein and peptide compounds of Actovegin appears in peak 4, with their molecular weight ranging from 4 to 45.2 kDA. The peak was also found in HCBF (1.11%). The results obtained indicate that there are qualitative differences between HCBF and Actovegin; however the differences found between them do not exclude that they share an active component. One might hypothesize that not only prevailing components, but also minor components in the composition may play some role in the biological activity of either medication [16]. In addition, the cumulative effect of all low-molecular components of either gel cannot be excluded. Table 2 demonstrates the amounts of some of the components found in HCBF versus Actovegin. A substantial difference in the content of the four hormones under study between HCBF and Actovegin was observed. Thus, the content of estradiol, a low-molecular hormone, in HCBF and in Actovegin was 4.650 pg/mL and 1.064 pg/mL, respectively. Estradiol is the most powerful natural estrogen and is produced mainly in the ovaries and placenta. Predominance of this hormone in the composition of HCBF could be explained by the source of HCBF [17]. The content of another steroid hormone, progesterone, in HCBF and in Actovegin was 0.129 pg/mL and less than 0.0005 pg/mL, respectively. The level of triiodothyronine in HCBF was 49-fold higher than that in Actovegin. The level of a low-molecular glucocorticoid, cortisol, in HCBF was statistically significantly different from that in Actovegin (3.430 nmol/l versus 0.341 nmol/l). The ions of calcium (ionized calcium), magnesium, phosphorus, and zinc (regulators of cellular homeostasis, nucleic acids and proteins, and energy metabolism) were found in HCBF [18, 19, 20], with the numbers of calcium, magnesium, phosphorus, and zinc ions being significantly higher than those in Actovegin. However, the number of phosphorus ions was the same as that in Actovegin. HCBF (but not Actovegin) was found to contain common chondroitin sulfates and total GAGs that reduce the inflammatory response to surgical trauma and can modulate the healing process without development of fibrosis [21]. Hexoses are involved in the metabolic pathways of the synthesis of biologically active molecules that are crucial for reparative regeneration processes, and the level of uronic acid with impurities like hexoses in HCBF was 4-fold higher than that in Actovegin. The level of glycoproteins in the comparator was 30.000 mg/l versus 0.750 mg/l in HCBF, and the difference was statistically significant. The glycoproteins found in blood as a response to stressors and the development of pathological processes exhibit immunomodulating activity and are capable of inhibiting proteinases [22]. Therefore, comparison of HCBF and Actovegin showed that they substantially differed from each other in composition both qualitatively and quantitatively. Our findings of great differences in the content of uronic acid and of the glycoproteins exhibiting a dominating effect on the metabolism of the connective tissue suppose that Oftocord gel and Actovegin gel may differ considerably from each other also in the nature of the effect they have on collagen synthesis. Based on the above, the research on the use of biological regulatory peptide-based therapeutic agents (including a low-molecular weight fraction of human cord blood) shows promise for accelerated corneal regeneration and prevention of leucoma after mechanical trauma to the eye. Conclusions First, in post-traumatic rabbit corneas treated with a HCBF-containing gel, the number of cells expressing CD95 was lower than in post-traumatic control corneas (p < 0.05) throughout the study period, which could be the consequence of prevailing restorative processes. Second, comparison of HCBF and Actovegin showed that they substantially differed from each other in composition (most of all, in the content of uronic acid and of the glycoproteins) both qualitatively and quantitatively, which explains the difference in collagen synthesis processes between the eyes treated with the gel containing HCBF and those treated with Actovegin gel.
Finally, normalization of collagen I and III production at the site of corneal damage in Oftocord gel-treated animals was observed early compared to Actovegin gel-treated animals, pointing to the potential for prevention of leucoma at the site at later time points after healing. References
|