J.ophthalmol.(Ukraine).2017;4:40-43.
|
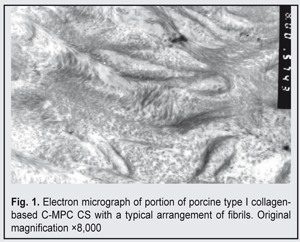
https://doi.org/10.31288/oftalmolzh201744043 Electron microscopy structure of collagen-based corneal substitutes N.I. Molchaniuk, Cand Sc (Med), O.I. Buznyk, Cand Sc (Med), N.E. Dumbrova, Dr Sc (Med), Prof., N.V. Pasyechnikova, MD, Dr Sc (Med), Prof. Filatov Institute of Eye Diseases and Tissue Therapy; Odessa (Ukraine) E-mail: a_buznik@bk.ru Purpose: To investigate the electron microscopy structure of biosynthetic corneal substitutes (CS) based on interpenetrating networks of collagen and 2-methacryloyloxyethyl phosphorylcholine (C-MPC). Materials and Methods: Transmission electron microscopy was used to investigate the structure of C-MPC CS made from 18% solution of recombinant human collagen (RHC) type III or porcine collagen (PCOL) type I. Results: Narrow (2-8 nm) collagen-like fibrils with a regular, mainly longitudinal orientation were found in the corneal substitutes. Although the ultrastructure and arrangement of these fibrils were typical for those of human corneal structure, they did not demonstrate some features of the human corneal structure. The fibrils from PCOL-MPC CS were somewhat wider and less regularly arranged than those from RHC-MPC CS; however, this did not effect the transparency of the implants. Conclusion: PCOL-MPC implants and RHC-MPC implants have as good optical properties as the human cornea, but their structure and arrangement of filaments are different from those of the human cornea, which may be associated with a very small size of the fibrils produced as a result of collagen molecule cross-linking; that is why they do not scatter light. Key-words: corneal substitute, donor cornea substitute, collagen, electron microscopy structure Introduction The potential for the fabrication of corneal substitutes through binding between collagen I or collagen III molecules has been demonstrated in recent years [1]. Using biochemical processes of collagen molecule cross-linking, we have managed to produce a graft that was similar to the human corneal stroma in terms of optical and mechanical properties. Others experimental studies have demonstrated that such a collagen matrix was a good substrate for corneal epithelial and nerve cell growth in vivo and in vitro [1]. In addition, a corneal stroma substitute fabricated from porcine collagen did not undergo rejection in rabbits [2]. Preliminary results of a Phase I clinical trial have confirmed experimental findings (no host rejection of transplanted collagen-based corneal substitute (CS)) and integration of CS into the corneal tissue of advanced keratoconus patients, with the nerves growing from the host into the graft, and with recipient epithelization of the graft [3]. Incorporation of an anti-inflamamtory syntheric phospholipid, 2-methacryloyloxy-ethyl phosphorylcholine (MPC), into CS, has lead to an improvement in its mechanical characteristics and to reduction in graft neovascularization in an animal model of alkaline ocular burn [4]. Initial clinical use of the collagen-MPC CS (C-MPC CS) demonstrated that these implants were safe and promising in patients with severe corneal pathologies in whom conventional donor cornea transplantation carried a high risk for rejection [5]. However, data are scarce on the ultrastructure of these implants, which evidences that they have been poorly understood. The purpose of this study was to investigate the microscopic structure of human or porcine collagen-based corneal substitutes with incorporated MPC. Materials and Methods Fabrication of C-MPC CS Collagen-based corneal substitutes were fabricated at the Biochemistry Laboratory of the Filatov Institute and at the Division of Cell Biology, Department of Clinical and Experimental Medicine, Faculty of Health Sciences, Link?ping University, through the development of two interpenetrating networks, (1) the first based on 18% aqueous solution of recombinant human collagen type III (RHC; FibroGen Inc., San Francisco, CA), or porcine collagen type I (PCOL; atellocollagen from Nippon Meat Packers, Inc., Tokyo, Japan, or TheraCol from Sewon Cellontech, Seoul, South Korea), with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and N-hydroxyl-succinimide used as cross-linking reagents, and (2) the second, an MPC (Paramount Fine Chemicals Co. Ltd, Dalian, China)–based network with poly(ethylene glycol) diacrylate (Sigma–Aldrich, Ontario, Canada), ammonium persulphate (Sigma-Aldrich), and N,N,N’,N’-tetramethyl ethylene diamine (Sigma-Aldrich) used as cross-linking reagents [5]. MPC with its cross-linkers were added to collagen solution during the synthesis phase. The gel obtained was cast between two glass plates with 250-500 ?m spacers. The gels were cured for 16 h at 100% humidity under nitrogen at room temperature. After demoulding, the final transparent hydrogels (C-MPC CS) were washed with Ringer’s solution, sterilized, and then stored in 1% chloroform solution at 4 °С to maintain sterility. Transmission electron microscopy of C-MPC CS Transmission electron microscopy was performed at the Pathology and Electronic Microscopy Laboratory of the Filatov Institute. Eight corneal substitutes, 6 mm in diameter and 500 ?m thick, were studied, including four RHC-MPC CS and four PCOL-MPC CS. Each CS was ?xed in 2.5% glutaraldehyde in phosphate buffer solution (pH 7.4), post-fixed with osmium tetroxide in the same buffer, dehydrated through a graded ethanol series, and embedded in an Epon/Araldite mix. Thereafter, ultra-thin sections were cut, stained with lead citrate according to the procedure described by Reynolds [8], and observed with a PEM-100-01 Transmission Electron Microscope (Selmi, Sumy, Ukraine). Results and Discussion C-MPC CS fabricated from porcine type I collagen The collagen bundles were arranged predominantly longitudinally. In the section, a small percentage of the bundles were arranged diagonally or orthogonally with respect to the microscope objective (Fig. 1).
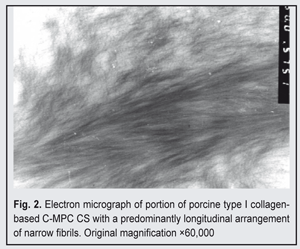
At high magnification (nearly 50,000x), the bundles appeared as composed of very fine (2-8 nm) collagen filaments; the outlines of these filaments were indistinct, and they showed no cross-striation (Fig. 2).
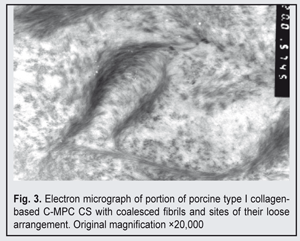
The fibrils tended to coalescence to form wider fibres. Isolated and loosely aggregated fibrils were seen at the periphery of the bundles. In some sections, there were sites with more densely packed fibrils, including loop-shaped aggregates of short and wider fibrils (Fig. 3).
C-MPC CS fabricated from type III recombinant human collagen In the sections, large areas were occupied by sites of homogeneous material within which homotypic and very narrow filaments with mainly longitudinal orientation could be distinguished. In some small sites, at high magnification, clusters of such loosely arranged narrow (2-5 nm) fibrils with indistinct outlines were revealed (Fig. 4A, 4B). Therefore, we conducted one of the first ever electron microscopy studies of C-MPC SS. The current study revealed the presence of collagen-like fibrils with a regular, mainly longitudinal orientation in the structure of the implants. Although the ultrastructure and arrangement of these fibrils were typical for those of human corneal structure, they did not demonstrate some features of the human corneal structure (e.g., they were not arranged in the lamellae characteristic of the human cornea) [1]. Our data are in accord with those of Hayes S. et al. [5], who also observed regularly oriented very fine collagen filaments in the ultrastructure of RHC-MPC corneal substitutes. The current study was the first to find that the ultrastructure of PORC-MPC implants was somewhat different from that of RHC-MPC implants. The collagen fibrils from the former were somewhat wider and less regularly arranged than those from the latter; however, this did not effect the transparency of the former implants. Conclusion Porcine collagen-MPC implants and RHC-MPC implants have as good optical properties as the human cornea, but their structure and arrangement of filaments are somewhat different from those of the human cornea, which may be associated with a very small size (2-8 nm) of the fibrils produced as a result of cross-linking of collagen molecules; that is why they do not scatter light. References
|