J.ophthalmol.(Ukraine).2017;4:55-59
|
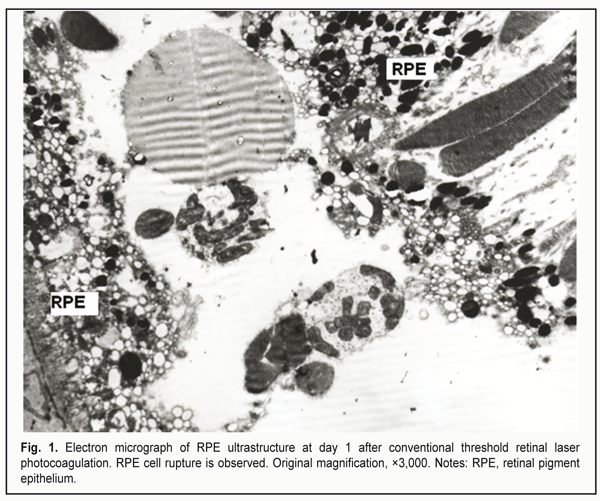
https://doi.org/10.31288/oftalmolzh201745559 Ultrastructural changes in the rabbit chorioretinal complex following 577-nm laser photocoagulation S.A. Fedchenko, Ophthalmologist, Post-grad Student, O.S. Zadorozhnyy, Cand Sc (Med), N.I. Molchaniuk, Cand Sc (Biol), Sen Res Fellow, A.R. Korol, Dr Sc (Med) Filatov Institute of Eye Diseases and Tissue Therapy; Odessa (Ukraine) E-mail: a_buznik@bk.ru Background: There is no unanimously adopted approach to titrating laser parameters in subthreshold retinal laser photocoagulation without ophthalmoscopically visible fundus changes. Purpose: To investigate the ultrastructural changes in the rabbit chorioretinal complex following 577-nm conventional, selective or micropulse retinal laser photocoagulation (RLPC). Materials and Methods: Four rabbits (8 eyes) were involved in the experimental study and underwent the RLPC modes with the 577-nm system (Supra 577). Rabbits (n = 2/time point) were euthanized at postlaser days 1 and 14. Ultra-thin sections of their ocular tissue specimens were subjected to electron microscopy. In addition, 2 intact rabbits (4 eyes) were used as controls for comparison. Results: Conventional RLPC resulted in damage to RPE cells, all photoreceptor compartments and choriocapillaries; selective RLPC resulted in damage to RPE cells and photoreceptor outer and inner segments; and micropulse RLPC with the power setting adjusted to 50% threshold resulted in damage mostly to apical aspects of RPE cells and photoreceptor outer segments. Conclusion: 577-nm micropulse RLPC with the power setting adjusted to 50% threshold is a more photoreceptor- and choriocapillaris-sparing approach compared to conventional and selective RLPC, and can be used in clinical practice as a laser treatment approach with the least invasive effect on the chorioretinal complex. Key-words: chorioretinal complex, laser radiation, ultrastructural changes, rabbit eye Introduction As new laser technologies for the treatment of fundus disorders emerge, theories are being developed to explain mechanisms of the effect of different modes of laser radiation on the intraocular structures. Photothermal mechanism of damage to fundus tissues is universal for infrared and visible laser radiation [1]. Conventional retinal laser photocoagulation (RLPC) is known to carry a risk of side effects and may be followed by visual field alterations, reduction in contrast sensitivity, and development of choroidal neovascularization. At present, it is considered that it is not necessary to damage all retinal layers in order to achieve therapeutic efficacy of laser. Subthreshold, or tissue-sparing, laser therapy is a promising alternative in the treatment of fundus disorders [2]. However, the issue of titration of laser parameters in tissue-sparing laser photocoagulation without ophthalmoscopically visible fundus changes has not yet been resolved. The purpose of this study was to investigate the ultrastructural changes in the rabbit chorioretinal complex following 577-nm conventional, selective or micropulse RLPC. Materials and Methods All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV dated 21.02.2006 and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), and approved by a local Bioethics Committee of the Filatov Institute. Four Chinchilla rabbits (8 eyes; weight, 2.5-3.5 kg) were included in this study, which was conducted at the Laser Department of the Filatov Institute. The animals were housed and bred conventionally. Each animal underwent biomicroscopy and ophthalmoscopy at baseline (before RLPC) and at day 1 after RLPC. In addition, two of them underwent biomicroscopy and ophthalmoscopy at day 14, immediately before they were euthanized. Prior to laser procedure, animals were anesthetized with thiopental sodium 10% (1.0 mL/kg, intramuscularly). Immediately thereafter, both eyes received a drop of proparacaine HCl (0.5%) for topical anesthesia. The pupils were dilated with atropine sulphate. After RLPC, a drop of sulfacyl natrium 20% and a drop of Ofloxacin 0.3% were applied to each eye four times daily. All procedures were performed by one surgeon, with central portion of Goldmann three mirror lens used to focus the aiming beam. The laser spot size was maintained at ~200 ?m. Rabbits (n = 2/time period) were euthanized at days 1 and 14 after laser treatment. The 577-nm Supra laser (Quantel Medical, Clermont-Ferrand, France) was used in the experimental study. It is an Optically Pumped Semiconductor Laser (OPSL) having a subthreshold micropulse mode, which generates customizable trains of microsecond pulses. To perform threshold photocoagulation, laser spots (exposure time, 0.1 s) were placed close together in a staggered pattern. Laser power was adjusted until grade 1 burn (according to the L'Esperance’s classification) was obtained at the site of laser exposure. In selective RLPC, a ten-pulse train was applied (10 ms ON, 100 ms OFF) until barely visible photocoagulation lesions were placed along myelinated fibers. Thereafter, laser power was reduced until no photocoagulation lesions could be detected within a minute after exposure to laser. In micropulse laser treatment, the laser was switched to the micropulse mode at 15% duty cycle (pulse train (burst time), 0.3 s; 0.17 ms ON, 1 ms OFF). Photocoagulation lesions were placed along myelinated fibers, and laser power was adjusted until grade 1 burn was obtained at the site of laser exposure, with a laser power reduction of 25%, 50% or 75%. Electron microscopy was performed at the Pathology and Electronic Microscopy Laboratory of the Filatov Institute. Electron micrographs of normal rabbit choroid and retina from 2 intact rabbits (4 eyes) were used for comparison. Each tissue specimen was ?xed in 2.5% glutaraldehyde in 1% phosphate buffer solution (pH 7.4), post-fixed with osmium tetroxide in the same buffer, dehydrated through a graded ethanol series, and embedded in an Epon/Araldite mix. Thereafter, ultra-thin sections were cut, stained with uranyl acetate and lead citrate, and observed, and photographed with a PEM-100-01 Transmission Electron Microscope (Selmi, Sumy, Ukraine). Results At day 1 after threshold RLPC with a yellow laser, dilated choriocapillaries and thinned endotehilal cells were observed. In addition, some cells of the retinal pigment epithelium (RPE) were destructed, and some of the membranes were vacuolated. The apical aspect of RPE cells with phagosomes and fragmented photoreceptor cell (PRC) outer segments was seen separated from mid-cell regions (Fig. 1). The interreceptor matrix (IRM) contained fragmented RPE cells, and photoreceptor cell outer (PRC OS) and inner segments (PRC IS).
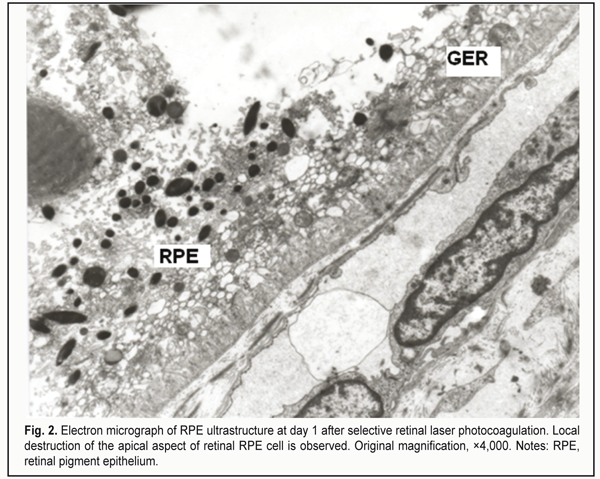
At day 1 after selective RLPC, disrupted RPE cells were observed within the laser irradiation focus. The mid- and apical cell regions were either lost or vacuolated. Content of choriocapillaries was compact, endothelial walls were thinned. The IRM was markedly edematous and contained fragments of PRC OS disks. RPE cells having vacuolated appearance, with membrane structures throughout the cytoplasm, and destructed apical aspect were seen near the above structures. Fragmented PRC OS were observed occupying spaces below mid-cell regions of maintained portions of the RPE cells (Fig. 2). At the laser irradiation focus, intercellular PRC edema extended to the external limiting membrane. Edema was less pronounced at the site adjacent to the focus and occupied mainly by PRC OS. Other PRC components like nuclei (PRC N) were not edematous.
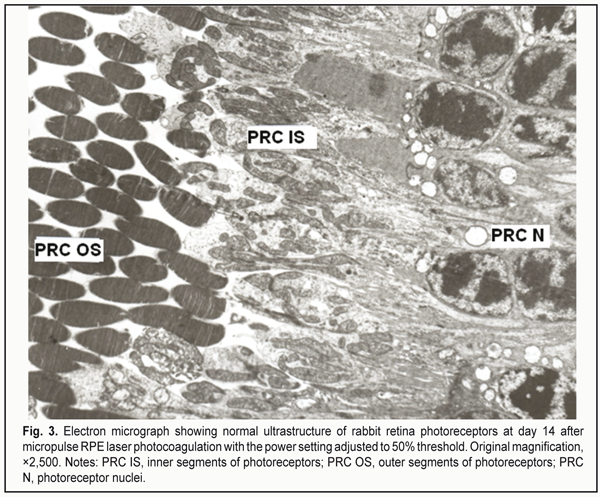
At day 14 after selective RLPC, the ultrastructure in most of the RPE cells was almost restored. The cytoplasm of these cells contained numerous organelles like ribosomes, polysomes, elements of granular endoplasmic reticulum (GER) and of smooth endoplasmic reticulum (SER), and mitochondria. In these cells, both apical aspects and apical microvilli contacting PRC OS were well developed. Compensatory and restorative processes in RPE cells were characteristic for the material taken at day 14. The PRC ultrastructure appeared to be mostly restored. At day 1 after micropulse RLPC with the power setting adjusted to 25% threshold, most of the RPE cells appeared nearly normal, and no PRC damage was observed. At day 1 after micropulse RLPC with the power setting adjusted to 50% threshold, most of the RPE cells and adjacent choriocapillaries (ChC) appeared nearly normal, whereas other RPE cells demonstrated damage to the apical aspect, decreased microvilli and phagosome abundance, and hydropic structural changes. In addition, some singular cells appeared destroyed while adjacent ones showed no such change in the photoreceptor layer. At day 1 after micropulse RLPC with the power setting adjusted to 75% threshold, some RPE cells showed damage to their mid- and apical regions, and some photoreceptors showed damage to their PRC OS. At day 14 after micropulse RLPC with the power setting adjusted to 25% threshold, the RPE cells appeared nearly normal, and the PRC demonstrated normal ultrastructure. At day 14 after micropulse RLPC with the power setting adjusted to 50% threshold, choriocapillaries showed some dilation and appeared nearly normal. Most of the RPE cells appeared nearly normal. Solitary cells in the RPE layer showed destruction, mostly, in the apical aspect, and exhibited hydropic and finely vesicular cytoplasmic changes. Nonetheless, most of the RPE cells demonstrated restoration of cell structure, and showed signs of viability (e.g., phagocytized fragmented PRC OS). All PRC components appeared well developed and had a normal ultrastructure (Fig. 3). At day 14 after micropulse RLPC with the power setting adjusted to 75% threshold, the RPE cells and PRC demonstrated damage to all cell components.
Discussion As far as we know, there is no unanimously adopted approach to performing micropulse retinal laser photocoagulation. Authors vary in approaches to applying subthreshold laser therapy with 577-nm laser [2-10]. Roider and colleagues [7-9] investigated the mechanism of the selective RPE damage induced by repetitive microsecond laser pulses in rabbits. They noted that selective damage to the RPE with sparing of adjacent structures can be obtained with laser pulses shorter than the time needed for heat conduction to spread temperature rise to adjacent sites, but long enough to induce damage to target structures. In addition, histological findings demonstrated substantial differences in outcomes between continuous wave (CW) laser photocoagulation of the retina and selective RLPC. Severe damage to the RPE was found in either of the two approaches used. In the RPE layer, the investigators observed damage to the RPE cells, with their subsequent replacement by a monolayer of new RPE cells. In addition, after retinal exposure to repetitive short laser pulses, the photoreceptors were arranged normally, and no damage to most of them was observed; the choriocapillaries remained unaffected, and intact red blood cells were observed. However, after conventional RLPC, the PRC were uniformly destroyed, and the choriocapillaries showed signs of damage. In contrast to CW photocoagulation, only minimal inflammatory response was found after selective RLPC, and macrophages, fragmented RPE cells, and lymphocytes were revealed in the damaged PRC layer [7-9]. Pasyechnikova et al [3] used selective (or subthreshold millisecond) RPE photocoagulation with millisecond pulses from a 532-laser in the treatment of diabetic macular edema and other fundus disorders. Lavinsky and Palanker [5] used a similar subthreshold RLPC approach for the treatment of chronic central serous chorioretinopathy (CSCR) with a 577-nm laser. The laser power was first titrated for a barely visible burn with 15-ms pulses, which was defined as 100% pulse energy. Treatment was then applied over the area of serous retinal detachment and adjacent nonthickened retina, using 30% pulse energy with the spot spacing of 0.25 beam diameter. Another titration protocol for adjustment of laser power and duration was used in the study of Yadav et al [9] of the treatment of CSCR with the 577-nm yellow micropulse laser. Initially, a continuous wave ‘test’ spot size of 100 mm, 0.2 s exposure time, and using enough power to cause mild retinal whitening was placed superonasally. Thereafter, they used similar settings, but with half the power, for CSCR treatment with micropulse RLPC. Romanova [4] suggested proceeding as follows: to titrate the power levels using a continuous wave, to activate the micropulse mode and to adjust the power by doubling it. However, this approach is associated with some problems, since it is difficult to predict how the laser parameter values will change following activation of the micropulse mode, with the values of two laser parameters (exposure time and power) changing simultaneously. Maia [6] also demonstrated the efficacy of 577-nm micropulse laser for CSCR, however, without mentioning his approach to titration of laser parameters for individual patient. In the study of Scholz et al [8], the individual power for the patient was titrated at a normal area of the retina, near the affected area in the monospot micropulse mode. The power titration was gradually increased until a just visible burn was seen. When this threshold was reached, the power was reduced by 50%. With this power, the subthreshold micropulse laser treatment of the affected retina was performed. We favor this approach to titration of laser parameters in the individual patient, since while adjusting the threshold laser power in the micropulse mode before switching to therapeutic treatment we adjust a single laser parameter (laser power). Therefore, with this approach, the effect of subthreshold micropulse laser applied in the absence of visible retinal photocoagulation lesions becomes more predictable. In our current electron microscopy study, at day 1 after micropulse RLPC with the power setting adjusted to 50% threshold, mild reactive changes were observed mostly in the apical aspect of RPE cells. However, these changes were found to be reversible, and practically vanished in the structures under investigation by day 14. Selective RLPC resulted in 1) more severe damage to RPE cells, which was more profound, up to destruction, and extended towards the choroid, and 2) damage to PRC OS without involvement of the nuclear compartment of the cell. This improved the potential for the restoration of PRC ultrastructure. In addition, a great capacity of RPE cells for compensatory and restorative processes (in particular, intracellular processes) was implemented at day 14. Conventional threshold laser photocoagulation resulted in the most severe and irreversible changes in the RPE cells, all photoreceptor compartments and choriocapillaries. Conclusion 577-nm micropulse RLPC with the power setting adjusted to 50% threshold is a more photoreceptor- and choriocapillaris-sparing approach compared to conventional or selective RLPC, and can be used in clinical practice as a laser treatment approach providing the least invasive effect on the chorioretinal complex. Availability of different 577-nm RLPC modes makes it possible to select the required extent of damage to the complex. Thus, conventional RLPC results in damage to RPE cells, all photoreceptor compartments and choriocapillaries; selective RLPC results in damage to RPE cells and photoreceptor outer and inner segments; and micropulse RLPC with the power setting adjusted to 50% threshold results in damage mostly to apical aspects of RPE cells and photoreceptor outer segments. At day 14 after micropulse RLPC with the power setting adjusted to 50% threshold, restoration of the ultrastructure of the chorioretinal complex was observed. References
|