J.ophthalmol.(Ukraine).2017;3:56-62.
|
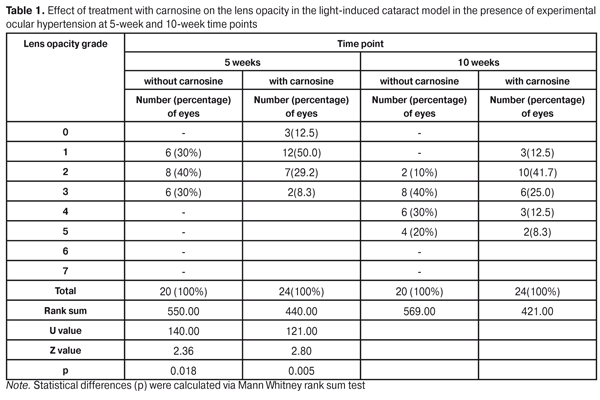
https://doi.org/10.31288/oftalmolzh201735662 Effect of treatment with carnosin on cataract development in experimental ocular hypertension I.N. Mikheitseva, Dr Sc (Med), Sen Res Fellow Moutasim Waleed A.R. Aldahdooh, Post-Grad Student S.G. Kolomiichuk, Res Fellow Iu.A. Zhuravok, Cand. Sc. (Med) Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine, Odessa, Ukraine E-mail: iradoc@mail.ru Background: Senile cataract and glaucoma are the major age-related and degenerative ocular disorders that can cause loss of vision and blindness. To date, the question of the possible mechanisms involved in the impact of the glaucomatous process on the lens is still open. Purpose: To investigate the effect and mechanism of action of carnosine on the light-induced cataract model in the presence of experimental ocular hypertension (OHP). Materials and Methods: Rabbits were exposed to light from mercury arc-discharge lamps (type DRF-1000, 1000 W; bandpass range, 350-1150 nm) for 9 hours a day during a 10-week period to investigate the effect of regularly instilled (twice daily during the same period) carnosine on the development of opacities and thiol levels in the lens in the light-induced rabbit cataract model. OHP was induced bilaterally by injecting 0.1 mL of 0.3% carbomer solution into the anterior chamber of both eyes. Results: Mild changes in lens clarity (grades 1 to 3) were more common in rabbits treated with carnosine, whereas more apparent changes in lens clarity were more common in untreated animals. At 10 weeks, grade 5 lens opacity was noted in 8.3% of the rabbits treated with carnosine and in 20.3% of the untreated animals. Introduction of carnosine in the rabbit model of light-induced cataract in the presence of OHP resulted in 49.2% increase in the reduced glutathione levels, 28.3% decrease in oxidized glutathione levels, 40.7% increase in the sulfhydryl (thiol) levels and 28% decrease in the disulphide levels in the lenses of treated experimental animals compared to untreated experimental animals. Conclusion: A long-term intraocular treatment with carnosine in the rabbit cataract model (a) improved the resistance of the lens to cataractogenic phototoxicity in the presence of ocular hypertension and (b) contributed to substantial normalization of thiol levels in the lens, thus preventing oxidative damage to proteins. Key words: ocular hypertension, age-related cataract, glutathione, thiol groups of proteins, lens, aqueous humor Introduction Age-related cataract is a leading global cause of blindness and visual impairment, and its social role has become ever more important due to the ageing of the world population [1, 2]. Currently, surgical treatment of cataract remains the only successful approach to restoration of vision in this disease. Surgical treatment of cataract is not, however, always pathogenetically substantiated. In addition, this approach is not always available (especially at locations with limited access to the appropriate medical infrastructure). Pharmacological treatment is undoubtedly the most available, economical and pathogenetically substantiated approach to cataract management. To date, however, none of the agents studied have been approved as preventing the progression of senile cataract. Developing such agents and investigating their efficacy is an important area for research [3-8]. Carnosine is a natural dipeptide (beta-alanyl-l-histidine) that is present at high levels, first and foremost, in such tissues as skeletal muscle and neural tissues. Its biological role in the body has not been fully elucidated. Carnosine has been shown to be a compound than can impact a variety of ageing-related processes and functions in the body. In 1994, McFarland and Holliday were the first to report on the retardation of the senescence of cultured human diploid fibroblasts by carnosine [7, 9]. The presence of endogenous carnosine in the lens suggests it is involved in normal physiological processes. Unlike carnosinase (the enzyme that breaks down carnosine into its amino acid components), carnosine synthase, the enzyme responsible for carnosine synthesis, has not yet been detected in the eye [10]. Carnosine, most probably, like most of nutrients, is delivered to the eye with systemic blood flow, diffuses into the aqueous humor, and, subsequently, directly into the cells of the lens. Carnosine peptides have been reported to exert the inhibitory effect on alpha-crystallin aggregates that cause lens opacification [11]. Babizhayev was the first to demonstrate the ability of carnosine to inhibit or reverse the formation of cataracts in cataract model induced by the administration of products of lipid peroxidation in rabbits [11]. Interestingly, that carnosine was found ineffective in preventing cataracts in streptozotocin-induced diabetic rats [12] or in an in vivo model of cataracts induced by sodium selenite [13]. More recently, carnosine was found to delay the progression of lens opacification in diabetic rats, but only during the earlier stages of the disease [14]. Based on the literature, one may state that there has been preliminary evidence for the role of carnosine in inhibition of the formation of senile cataract. Despite rather homogeneous reports from various investigators on the beneficial effect of carnosine on the lens, further studies of the mechanism of action of this natural non-toxic agent in cataractogenesis are mandated. To the best of our knowledge, there have been no reliable reports in the literature on the use of carnosine in glaucoma. However, since some of the mechanisms (e.g., oxidative stress) involved in the development of cataract are similar to those involved in the development of neurodegenerative diseases in general (and glaucoma, in particular), it might be feasible to use this dipeptide in the treatment of glaucoma. Thus, the following is known regarding its use as a neuroprotector. In a model of focal cerebral ischemia following middle cerebral artery occlusion in mice, carnosine treatment significantly decreased infarct size and neuronal damage [15]. The authors believe that the neuroprotective effects of carnosine after focal ischemia may be mediated in part through its known antioxidant properties, with preservation of normal level of glutathione in the brain of treated mice. In addition, carnosine is known to penetrate the blood-brain barrier readily [16]. The authors concluded that carnosine may offer pluripotent beneficial effects by decreasing oxidative stress and by regulating MMP activity, further indicating its neuroprotective potential against ischemic injury. This conclusion pertains not only to the potential to inhibit multiple mechanisms of injury after ischemia, but also to safety and nontoxocity of this compound when compared with alternative candidate treatments that block only a single pathway. Studies of eye disease models involving animals receiving carnosine to correct for the induced pathological changes would shed light on the possibility and feasibility of using this natural compound as a therapeutic means in the management of cataract associated with glaucoma. Therefore, the study purpose was to investigate the impact and mechanism of action of carnosine in the light-induced cataract model in the presence of experimental ocular hypertension. Materials and Methods A total of 34 Chinchilla rabbits (weight, 2.5–3.2 kg) were used in the study. All animal experiments were conducted in accordance with the General Ethical Principles of Animal Experiments (approved by the Third National Congress on Bioethics Ukraine, Kyiv, 2007) and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986). Light was used to induce cataract in the presence of experimental ocular hypertension in animals of Group 1 (10 rabbits) and Group 2 (12 rabbits). The rabbits of Group 2 also received a course of treatment with carnosine. Intact animals (12 rabbits) were used as controls. To reproduce a light-induced cataract (LIC) model [17], animals of Groups 1 and 2 were exposed to light from two mercury arc-discharge lamps (type DRF-1000, 1000 W; bandpass range, 350-1150 nm) for 9 hours a day during a 10-week period. Ocular hypertension (OHP) was induced bilaterally in animals of Groups 1 and 2 by injecting 0.1 mL of 0.3% carbomer solution into the anterior chamber of both eyes [13, 14]. Animals were anesthetized generally with ketamine (50 mg/kg), and locally with 0.5% procaine hydrochloride instilled into the conjunctival sac. Carnosine was introduced bilaterally in animals of Group 2 by instilling 0.05 mL of 0.3% solution into the conjunctival cavity of both eyes, twice daily during a 10-week period. Lens opacities were classified and graded 1 to 7 with the Oxford Clinical Cataract Classification and Grading System (OCCCGS) [18]. To determine the reduced and oxidized glutathione levels in lens, the lens homogenate was prepared at a 1:7 (w/v) ratio with 6% perchloric acid. In addition, 6% perchloric acid was added to the aqueous humor at a 1:1 (v/v) ratio. The lens homogenate was then centrifuged for 10 min at 1,000 g at 5°C, and the deproteinized supernatant was neutralized with potassium phosphate (1.75 mole/l). The neutral solution thus obtained was centrifuged for 10 min at 3,000 g. The reduced and oxidized glutathione levels in the supernatant obtained were determined. To determine the sulfhydryl and disulphide levels in the lens, the lens homogenate was prepared at a 1:9 ratio (w/v) with 0.9% sodium chloride. Native aqueous humor was diluted at a 1:1 ratio with 0.9% (v/v) sodium chloride. The extracts obtained were then centrifuged for 10 min at 10,000 g at 5°C. The sulfhydryl and disulphide levels were determined in the protein-rich supernatant thus obtained. Reduced and oxidized glutathione levels, and thiol and disulphide levels in lens proteins were determined spectrophotometrically. When the concentration of the reduced glutathione is being determined, glyoxalase I converts methylglyoxal and reduced glutathione to S-lactoylglutathione that has an absorption maximum at 240 nm [19]. When the concentration of the oxidized glutathione is being determined, reduced nicotinamide adenine dinucleotide phosphate (NADPH) is oxidized as a result of enzymatic reduction of oxidized glutathione, and the decrease in the optical density is monitored spectrophotometrically at 340 nm [14]. The method for determination of the level of sulfhydryl groups of proteins consists in determination of the amount of thionitrophenylate anion released following reaction of 5,5'-dithiobis(2-nitrobenzoic acid) with free sulfhydryl groups of proteins. Disulfide groups of proteins were reduced to sulfhydryl groups by treatment with dithiothreitol. The number of disulfide groups in the protein was determined through comparison of the levels of free thionitrophenylate anion before and after treatment with dithiothreitol. The coefficient of variation of the method is 1.02% [20]. Statistical analyses were completed with SPSS software version 11.0 (SPSS Inc., Chicago, IL, USA). The Mann-Whitney U test was used to assess the development of lens opacities. The unpaired t-test was used for intergroup comparison of biochemical data. Results and Discussion The mean IOP values in Group 1 (untreated LIC and OHP), Group 2 (treated LIC and OHP) and controls were 26.5±1.4 mm Hg, 20.1±1.8 mm Hg, and 15.2±0.7 mm Hg, respectively. Initially, carnosine’s ability to enhance the resistance of the lens to damage from irradiation and elevated IOP was investigated. At 5 weeks and 10 weeks, mild changes in lens clarity (grades 1 to 3) were more common in rabbits treated with carnosine, whereas more apparent changes in lens clarity were more common in untreated experimental animals (Table 1).
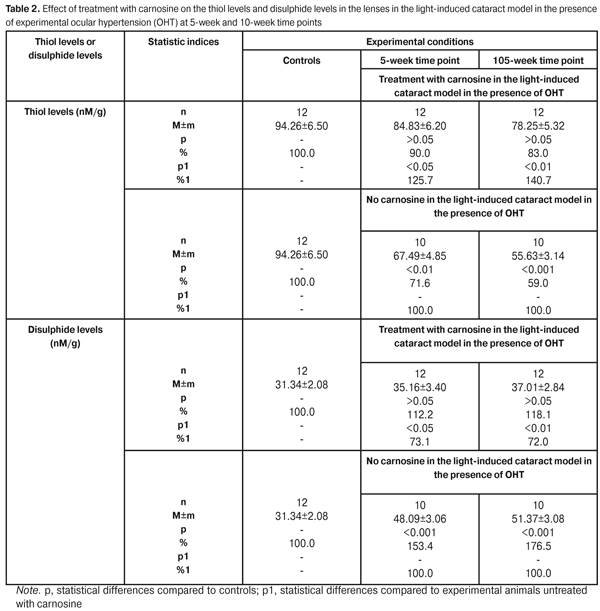
Treatment with carnosine resulted in the reduction of light-induced cataractogenic effect in the presence of developing OHP. Thus, by the 10-week time point, the number of eyes with grade 5 lens opacity in the treated experimental group was 2.5 times lower than that in the untreated experimental group. No statistically significant abnormal lens changes were found in intact rabbits. Oxidative stress is a major mechanism involved in the development of senile cataract as well as primary glaucoma. Oxidation of protein molecules with cleavage of sulfhydryl bonds followed by formation of disulphide bonds instead of broken sulfhydryl bonds is one of the stages of oxidative stress. To assess the effect of carnosine on the level of oxidative stress in the anterior eye in light-induced cataract in the presence of OHT, first of all, the thiol-disulphide system in the lens was investigated. With this in mind, the sulfhydryl (thiol) and disulphide levels in lenses of experimental animals were determined. In addition, the effect of carnosine on reduced and oxidized glutathione levels in the lens was investiaged. Table 2 presents the data on the thiol and disulphide levels in lenses of experimental animals affected by light and OHP.
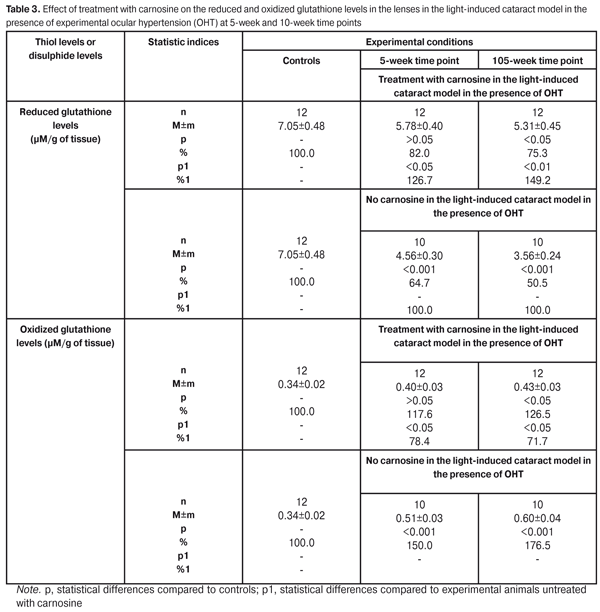
At the 10-week time point, the thiol levels in the lenses of animals treated with carnosine averaged 83% (p > 0.05) of those in controls and 140.7% (p1 <0.01) of those in untreated experimental animals. At the same time, the thiol levels in the lenses of untreated experimental animals averaged 59% (p < 0.001) of those in controls. The pattern of changes in the disulphide levels in lenses of experimental animals was somewhat different. At the 10-week time point, the disulphide levels in the lenses of untreated experimental animals were markedly increased and averaged 176.5% of those in controls, whereas the levels in the lenses of animals treated with carnosine averaged 118.1% (p > 0.05) of those in controls and 72.0 % (p1 <0.01) of those in untreated experimental animals. Therefore, introduction of carnosine in the rabbit model of cataract in the presence of OHP resulted in substantial normalization of protein thiol levels in the lens, thus preventing oxidation of thiol groups, which evidenced reduced breakage of protein molecules. Under these conditions, there was an obvious reduction in the protein disulphide levels in the lens of experimental animals, which confirmed the conclusion that the rate of destruction of proteins in the lens reduced. The next stage of the study was to investigate the reduced glutathione system in the lenses. With this in mind, reduced glutathione levels were investigated (Table 3). At the 10-week time point, the reduced glutathione levels were 49.5% lower in the lenses of experimental animals treated with carnosine than in the lenses of controls. At the same time, the oxidized glutathione levels were 76.5% higher in the lenses of experimental animals treated with carnosine than in the lenses of controls.
The long-term carnosine treatment course in a rabbit model of light-induced cataract in the presence of OHT had a stabilizing effect on the glutathione defense system in the lens. In experimental animals treated with carnosine, the reduced glutathione levels were significantly increased, whereas the oxidized glutathione levels were significantly decreased as compared to untreated experimental animals. Thus, at the 10-week time point, the reduced glutathione levels in the lenses of treated experimental animals averaged 75.3% (p < 0.05) of those in controls and 149.2 % (p1 <0.01) of those in untreated experimental animals. At the same time, the oxidized glutathione levels in the lenses of treated experimental animals averaged 126.5% (p < 0.05) of those in controls and 71.1 % (p1 <0.05) of those in untreated experimental animals. The changes in the reduction potential of the glutathione system similar to those in the lens tissue were noted in the aqueous humor of experimental animals. The reduced glutathione levels in the aqueous humor of untreated experimental animals showed a decrease over the study period, and, at the 10-week time point, averaged 56.2% (p < 0.001) of those in controls. At the same time, the oxidized glutathione levels in the aqueous humor were 52.5% higher in untreated experimental animals compared to controls (p <0.01). In the aqueous humor of treated experimental animals, the reduced glutathione levels at 5-week and 10-week time points were 15.4 % (1.37±0.07 ?M/l) and 21.0% (1.28±0.10 ?M/l), respectively, decreased compared to controls (1.62±0.09 ?M/l). At 5-week and 10-week time points, the oxidized glutathione levels in the aqueous humor of treated experimental animals were 7.5% (0.43±0.03 ?M/l) and 22.5% (0.49±0.10 ?M/l), respectively, increased compared to controls (0.40±0.09 ?M/l). In addition, at 5-week and 10-week time points, in treated experimental animals, the reduced glutathione levels were 20.2% and 40.7%, respectively, increased, whereas the oxidized glutathione levels were 20.4% and 19.7%, respectively, decreased, compared to untreated experimental animals. Therefore, we obtained experimental data on the beneficial effect of carnosine on the lens glutathione and thiol-disulphide systems damaged by light-induced cataract and under elevated IOP conditions, and these data help identify an important component in the mechanism of accelerated development of alteration in the optical properties of the lens. In our opinion, alterations in glutathione defense in the lens, if aggravated by ocular hypertension, are a key pathochemical mechanism of cataractogenesis. Effective contribution of carnosine to a significant improvement in the imbalance between oxidized and reduced glutathione in the lens, and to restoration of the thiol-disulfide system suggests feasibility of using this agent for prevention and treatment of cataract development. Conclusions First, a long-term intraocular treatment with carnosine in the rabbit cataract model improved the resistance of the lens to cataractogenic phototoxicity under ocular hypertension. By the 10-week time point, 8.3% of eyes in the treated experimental group had grade 5 lens opacity was versus 20.0% in the untreated experimental group. Second, introduction of carnosine in the rabbit model of light-induced cataract in the presence of OHP resulted in activation of the glutathione defense system in the lens, with 49.2% increase in the reduced glutathione levels and 28.3% decrease in oxidized glutathione levels in the lenses of treated experimental animals compared to untreated experimental animals. Finally, in the rabbit model of light-induced cataract in the presence of OHP, treatment with carnosine hampered activation of oxidative damage to proteins, with 40.7% increase in the sulfhydryl (thiol) levels and 28% decrease in the disulphide levels in the lenses of treated experimental animals compared to untreated experimental animals. References
|