J.ophthalmol.(Ukraine).2017;3:9-14.
|
https://doi.org/10.31288/oftalmolzh20173914 Efficacy assessment of treatment of patients with stage III primary open-angle glaucoma using a combined method (phosphene electrical stimulation and photomyostimulation) V.A. Putienko V.S. Ponomarchuk, Dr.Sc. (Med.), Prof. Filatov Institute of Eye Diseases and Tissue Therapy Odessa, Ukraine E-mail: alputienko@yandex.ru
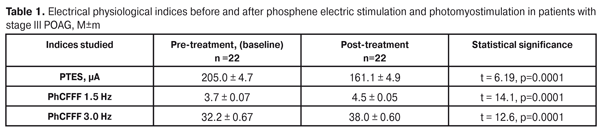
The purpose: to assess the efficacy of a combined method, including phosphene electric stimulation (FES) and photomyostimulation, in treatment of patients with stage III primary open-angle glaucoma (POAG) with compensated IOP. Material and Methods. Twenty-two eyes (22 patients) with stage III POAG with drug-compensated IOP were studied. The combined method consisted of a 10-minute FES session and a 10-minute photomyostimulation session. A patient had a 20-minute rest between the sessions. The treatment course included the10 sessions. To perform the FES session, a current value was specified in each certain case depending on a baseline phosphene threshold of electric sensivity (PTES). Photostimulation was carried out with impulse movement frequency (IMF), optimal to a patient, in a chaotic mode under scotopic conditions. Results. The treatment resulted in the improvement of the functional mobility of the oculomotor system (the values of the IMX index significantly increased in all three modes). The functional activity of the visual system increased: PTES decreased by 21.4%, PhCFFF in the 1.5 and 3.0 modes increased by 21.6% and 18.1%, respectively. The mean deviation of light sensitivity decreased by 14.6% and the mean retinal light sensitivity according to all MS threshold values increased by 17.9 % with enlarged summarized visual field by14.8%. The method proposed had a positive effect for ocular hemodynamics. Thus, the facility of aqueous outflow increased by 29.4%, the Ро/С coefficient decreased by 27.1% and the rate of aqueous flow increased by 31.1% Conclusion. The proposed method of treatment can be included in the complex of curative measures for the patients with stage III POAG in order to increase the functional activity of the visual system and to improve the patients’ quality of life. Key words: primary open-angle glaucoma, treatment, phosphene electric stimulation, photomyostimulation Background Glaucoma is the second leading cause of blindness globally [8]. Experts have estimated that 8.4 million had glaucoma-caused blindness that comprises 21% of total blindness worldwide. It is suggested that the number of patients with this disease will increase to 79.6 million people by 2020 [10]. Primary open-angle glaucoma (POAG) is a progressive neurodegenerative disease of the eye that is characterized by loss of retinal ganglion cells and their axons, which leads to neuropathy of the optic nerve with apparent visual field loss. In advanced stages of the disease, the progression process includes neuron loss in the lateral geniculate body and visual cortex [6]. An effective decrease of intraocular pressure (IOP), which presently is achieved by an ocular hypotensive drug combination in more than 80% of cases, considerably suppresses progressive suffering of the nerve tissue and enables to preserve visual acuity [9]. Thereafter, in the foreground of the treatment enters the improvement of functional activity of nerve elements remained in the retina. This is of a special importance for advanced (stage III) glaucoma when apparent retinal ganglion cell loss occurs against the thinning of the nerve fiber layer with the thinning of papillomacular bundle fibers [5, 7]. Apart from significant visual field loss, the patients with advanced POAG have a significantly decreased functional mobility of the oculomotor system [3]. It has been shown that stimulation of the oculomotor system of the patients with advance glaucoma using a photomyostimaulation method enables to significantly increase the oculomotor system functional activity in indices of impulse movement frequency (IMF) in all modes as well as to improve the visual system lability in indices of critical flicker fusion frequency (CFFF) and critical flicker appearence frequency (CFAF) [3]. One of the methods for glaucoma neuropathy treatment is phosphene electric stimulation (FES). FES improves the activity of the visual system through improving blood supply in the eye and cortex visual centers [1, 4]. Using the FES method in advance glaucoma patients have been shown to significantly increase the functional activity of the inner retinal layers with notablely improved retinal light sensitivity [2]. A combined method of FES and photomyostimulation in treatment of patients with stage III (advanced) glaucoma has not been performed yet, which was the background for the present study. The purpose: to assess the efficacy of the combined method of FES and photomyostimulation in treatment of patients with stage III glaucoma patients with compensated IOP. Materials and Methods Twenty-two eyes (22 patients) with stage III POAG with drug-compensated IOP were studied. Diagnosis was made using data of ophthalmoscopy, gonioscopy, electron tonography, computed static perimetry, and optical coherence tomography (OCT). OCT was performed using Carl Zeiss (CIRRUS Photo 800) and a mean thickness of retinal nerve fiber layer (RNFL) was recorded. The age of patients averaged 68.4±0.9 years. Visual acuity ranged from 0.1 to 0.5 and averaged 0.31±0.03. Optic disc cupping was enlarged in all cases and reached the rim of the disc in 19 eyes (86.4%). OCT revealed the mean RNFL thickness equal to 56.1±0.91mm, ranged from 49 mm to 64 mm, and the mean cup-to-disc ratio equal to 0.86±0.007, ranged from 0.81 до 0.91. Computed static perimetry was performed using Oculus vision field analyzer (TOPCON) with Glaucoma treshold, 30-2 Fast threshold. Mean deviation (MD) of the retinal light sensitivity from the norm was computed and ranged from 12.6 dB to 15.0 dB with the mean value of 13.64±0.13 dB. Mean light sensitivity (MS) in all determined threshold values ranged from 2.91 to 6.52 with the mean value of 4.51±0.20. In all cases, a concentric visual field constriction in one or more segments reaching 15° from the fixation point was noted. Baseline summarized visual field according to eight quadrants was 243.7±7.09° within the range of 180° -290°. Tonography was performed using the A.P.Nesterov method. Baseline tonography data were as follows: intraocular pressure, Р0 – 16.1±0.2 mmHg; the facility of aqueous outflow, С – 0.17±0.003 mm3/min/mmHg; the rate of aqueous flow, F – 1.70±0.02 mm3/min; a P0/C coefficient 100.99±2.1. A FOSFEN-1 unit was used to determine a phosphene threshold of electric sensitivity (PTES) and phosphene critical flicker fusion frequency (PhCFFF) in 1.5 and 3.0 modes; treatment sessions were also performed using the FOSFEN-1 unit. Photomyostimulation was performed using an ophthalmic photomyostimulator unit (FMS-1). The combined method consisted of a 10-minute FES session and a 10-minute photomyostimulation session. A patient had a 20-minute rest between the sessions. The treatment course included the 10 sessions. To perform the FES session, a current value was specified in each certain case depending on the baseline PTES level. Photostimulation was carried out in impulse movement frequency (IMF), optimal to a patient, in a chaotic mode under scotopic conditions. The patient followed the movements of a test object for one minute and, then, had a one-minute rest. To assess the treatment success, we recorded: changes in PTES, PhCFFF in the 1.5 and 3.0 modes; changes in the oculomotor system functional mobility in IMF, Hz, in three kinetic modes: horizontal (H), vertical (V), and chaotic (C); and changes in the visual system lability in critical flicker fusion frequency (CFFF), Hz, and critical flicker appearance frequency (CFAF), Hz, in three kinetic modes (H, V, C) and a static (motionless) mode with a central fixation point. Also, post-treatment computed static perimetry and tonography data were assessed. Statistic data processing was performed using STATISTICA 7.0 software package. A pairwise Student (t) criterion was used for data analyses. Critical p-level when testing statistical hypothesis was equal to 0.05. Results Using the combined method made it possible to notably improve the functional activity of the inner retinal layers: the mean value of PTES decreased after treatment from 205.0±4.7 to 161.1±4.9 µA, i.e. by 21.4% (p=0.01) (Table 1).
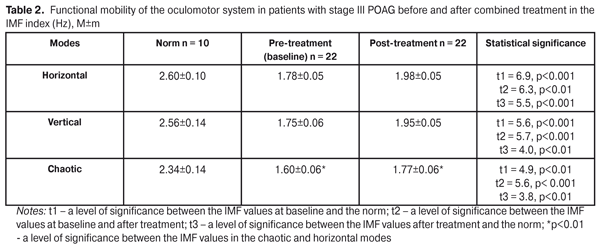
The activity of the papillomacular bundle was also improved: the PhCFFF values statistically significantly increased both in the 1.5 and 3.0 modes from 3.7±0.07 to 4.5±0.05 Hz, i.e. by 21.6% (р = 0.0001) and from 32.2±0.67 to 38.0±0.6 Hz, i.e. by 18.1% (р = 0.0001), respectively (Table 1). Afterwards, we studied the functional mobility of the oculomotor system. The mean value of IMF in H-mode increased by 11.2% (р < 0.001): 1.78±0.05 Hz at baseline vs. post-treatment 1.98±0.05 Hz. In V-mode the mean IMF value increased by 11.4 %: from pre-treatment 1.75±0.06 Hz to post-treatment 1.95±0.05 Hz (р<0.001). The IMF value in C-mode averaged 1.60±0.06 Hz at baseline and significantly increased to 1.77±0.06 Hz (p<0.001), i.e. by 10.6%, after treatment. Comparison of the data showed that the mean IMF value in C-mode both at baseline and after treatment was significantly lower than that in H-mode (Table 2).
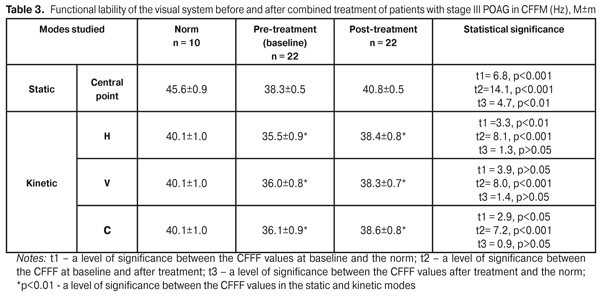
Thus, the 10 FES sessions, performed in combination with photomyostimulation in patients with stage III POAG, made it possible to significantly increase the mobility of the oculomotor system in the IMF index in all three modes. However, the IMF values in the three modes remained far below the norm even after treatment performed. Data on the lability of the visual system at baseline after combined treatment in the CFFF index are given in Table 3.
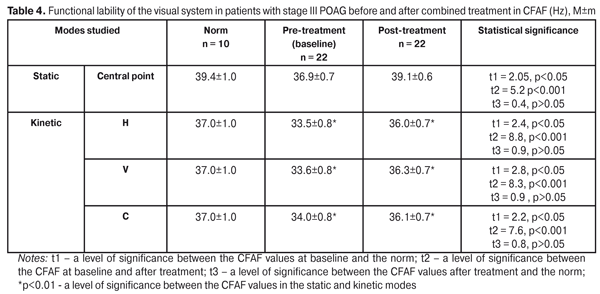
The CFFF index of the visual system lability in the static mode (central point of fixation) at baseline and after treatment was 38.3±0.5 Hz and 40.8±0.5 Hz, respectively, having increased by 6.2 (р<0.001). CFFF in the kinetic H-mode was 35.5±0.9 Hz and 38.4±0.8 Hz at baseline and after treatment, respectively, i.e. significantly increased by 7.6%. The V-mode CFFF value equaled 35.8±0.8 Hz and 38.6±0.7 at baseline after treatment, respectively, i.e. increased by 7.3%. The CFFF value in the C-mode was significantly increased by 6.5%: from 36.1±0.9 Hz at baseline to 38.6±0.8 Hz after treatment. In the static mode, the CFFF value after treatment remained far below the norm while all the post-treatment values in the kinetic mode did not differ significantly from the norm (p>0.05). It should be noted that, both at baseline and after treatment, the mean values of CFFF in kinetic H-V-C-modes were significantly lower than those in the static mode (Table 3). We determined the functional lability of the visual system according to the CFAF index before and after combined treatment. The mean CFAF value in the static mode was 36.9±0.7 Hz at baseline vs. 39.1±0.6 after treatment, i.e. increased by 5.7% (р<0.001). The kinetic H-mode CFAF value also significantly increased by 6.9 %: from pre-treatment 33.5±0.8 Hz to post-treatment 36.0±0.7 Hz (р<0.001). The kinetic V-mode CFAF value equaled 33.6±0.8 Hz at baseline, increased by 7.4% after treatment comprising 36.3±0.7 Hz (р<0.001). The C-mode CFAF was 34.0±0.8 Hz and 36.1±0.7 Hz at baseline and after treatment, respectively, the increase was by 5.8%. All values after treatment did not differ significantly from the norm (р>0.05). Herewith, the mean values of CFAF in the kinetic H-V-C-modes were significantly lower than those in the static mode (Table 4).
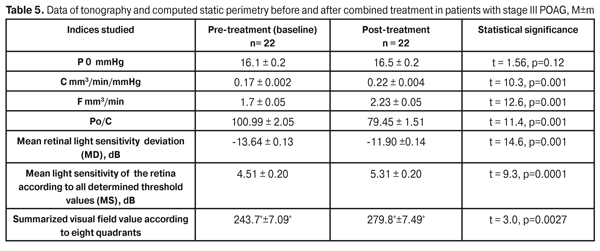
So, the combined treatment course being performed in patients with stage III POAG, the functional lability of the visual system significantly improved and did not differ from the norm in all indices studied, apart from the PTES index in the static mode. This gives evidence that the combination of FES and photomyostimulation has a positive effect on the lability function of the visual system in patients with stage III POAG. Also, we analyzed the state of ocular hemodynamics at baseline and after the combined treatment using the method of FES and photomyostimulation. The mean value of IOP (P0) did not change significantly: 16.1±0.2 mmHg vs.16.5±0.2 mm Hg, at baseline and after treatment, respectively. The mean value of the facility of aqueous outflow (C) increased after treatment by 29.4% (р = 0.001): from 0.17±0.002 at baseline to post-treatment 0.22±0.004 mm3/min (Table 3). The Ро/С coefficient significantly improved by 27.1%: 100.99±2.05 vs. 79.45±1.51, at baseline and after treatment, respectively (р = 0.001). By 31.1% increased the rate of aqueous flow (F): from 1.7±0.05 to 2.23±0.05 mm3/min, respectively (р=0.001). The data obtained demontstrate an apparent positive effect of the therapy. The combined treatment resulted in significantly improved data of the computed static perimetry data. The mean deviation of light sensitivity improved by 14.6% (р = 0.001), from -13.64±0.13 dB to -11.90±0.14 dB; and the mean retinal light sensitivity according to all MS threshold values increased from 4.51±0.22 dB to 5.31±0.23 dB, i.e. by 17.9% (р = 0.034). Also, a significant increase was noted in summarized visual field according to 8 quadrants: from pre-treatment 243.7°±7.09° to post-treatment 279.8°± 7.49°, i.e. by 14.8% (р=0.0041) (Table 5). At the same time, the therapy performed did not influence significantly on the visual acuity. The mean visual acuity value equaled 0.31±0.03 and 0.33±0.03, at baseline and after treatment, respectively (р=0.56).
Conclusion Thus, the combination of FES and photomyostimulation made it possible to significantly increase the functional activity of the visual system. PTES decreased by 21.4%, PhCFFF in the 1.5 and 3.0 modes increased by 21.6% and 18.1%, respectively. The mean deviation of light sensitivity decreased by 14.6% and the mean retinal light sensitivity according to all MS threshold values increased by 17.9 % with summarized visual field enlarged by 14.8%. The treatment resulted in the improvement of the oculomotor system functional mobility according to the IMF index in all three modes and of the visual system lability according to the static and kinetic CFFF and CFAF indices. The method proposed had a positive effect on ocular hemodynamics. Thus, the facility of aqueous outflow increased by 29.4%, the Ро/С coefficient decreased by 27.1%, and the rate of aqueous flow increased by 31.1%.
On the data given, the proposed method of treatment can be included in a complex of curative measures for patients with stage III POAG in order to increase the functional activity of the visual system and to improve the patients’ quality of life. References
|