J.ophthalmol.(Ukraine).2017;3:63-69.
|
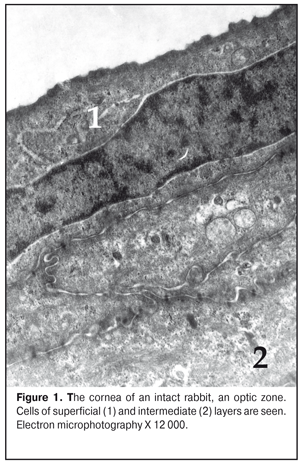
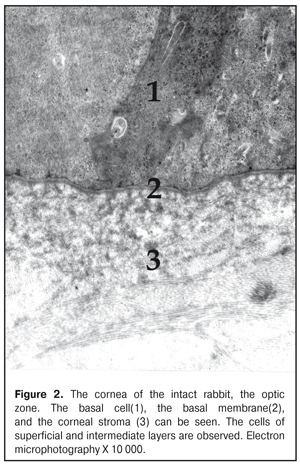
https://doi.org/10.31288/oftalmolzh201736369 Effect of colloidal silver nanoparticle solution instillation on the ultrastructure of the corneal epithelium and stroma V.A. Ulyanov, Prof., Dr. Sc. (Med.) 1 M.B. Makarova, a Postgraduate student, immunologist1, 2 N.I. Molchaniuk, Cand. Sc. (Biol.) 2 N.A. Ulyanova, Prof., Dr. Sc. (Med.) 1 V.M. Skobeeva, Cand. Sc. (Phys.-Math.) 3 E.A. Chernezhenko, a junior research fellow 2 1 Odessa National Medical University; 2 Filatov Institute of Eye Diseases and Tissue Therapy 3 Mechnikov Odessa National University Odessa, Ukraine E-mail: makarovambdoc@mail.ru Introduction. Existing methods of treatment for bacterial keratitis do not always provide a positive therapeutic effect and do not prevent the occurrence of relapses. The main reason for this is the development of resistance of microorganisms to antibiotics. The use of silver nanoparticles with antimicrobial properties may be promising in the treatment of bacterial keratitis. However, the effect of silver nanoparticles on the structure of the cornea has not been adequately studied. Objective: to study the effect of instillations of a colloidal solution of silver nanoparticles measuring 30 nm on the ultrastructure of the epithelium and stroma of the rabbit's cornea. Material and Methods. The colloidal solution of silver nanoparticles 30 nm in size was instilled into the conjunctival cavity of rabbits three times a day. Ultrastructural changes in epithelial cells and corneal stroma, changes in connective tissue plates and collagen fibrils of the stroma were evaluated at Days 15 and 30 of the experiment. Results. The effect of silver nanoparticles caused an increase in the number of ribosomes in the superficial cells of the corneal epithelium, hydrophilic changes in membrane organelles, an increase in the number of ribosomes in all cells of the basal layer, and destruction of individual organelles in single cells. In the stroma, the edema of the main substance was observed; the areas of defibration, homogenization or fragmentation of collagen fibril bundles were determined. The dystrophic changes were more severe as the duration of instillations of silver nanoparticles increased. Conclusions. The daily instillations of the colloidal solution of silver nanoparticles measuring 30 nm in the conjunctival cavity in the course of 15 and 30 days caused the dystrophic changes in the superficial and basal cells of the corneal epithelium. In the stroma of the cornea, the dystrophic changes in keratocytes, violation of the architectonics of connective tissue plates, defibration and fragmentation of collagen fibrils were detected. Key words: corneal ultrastructure of the epithelium and stroma, silver nanoparticles, experiment Background Treatment of bacterial keratitis is one of the current issues in ophthalmology today. The disease is characterized by a severe clinical course which leads to vision impairment as well as to complication development [1]. Existing methods of treatment for bacterial keratitis do not always provide a positive therapeutic effect and do not prevent the occurrence of relapses [2]. The main reason for this is the development of resistance of microorganisms to antibiotics, resulting in appearance of superstrains and disbacteriosis development. However, colloidal solutions of silver nanoparticles, which have an anti-microbial effect, in a form of Collargol and Protargol drugs are actively used in ophthalmology. The drugs pointed are products of compounds of colloidal (metallic) silver with protein. Bacterial keratitis pathogenesis is a complicated process involving the interaction of many local and general factors (hormonal, paracrine, nervous, vascular, and cellular ones) [3]. In this regard, a further search for therapeutic agents than could be used in keratitis treatment is required. Today, investigations on the effect of silver nanoparticles (AgNPs) on the local ocular immunity which plays an important role in the inflammation course are of a special scientific and practical interest [4]. AgNP application is promising in bacterial infections complicated by biofilm formation. AgNPs have been noted to have an immune-modulating action; it has been determined that silver is able both to stimulate and to inhibit a neutrophil phagocytic activity in dependence on a doze and a size of silver nanoparticles [5]. AgNPs have anti-microbial and anti-inflammatory characteristics [6]. However, taking into consideration such a small size and high penetrability of AgNPs, not all authors share the opinion that they are completely safe for the medical application [7, 8, 9]. So, this requires the further studying AgNPs in regard of their application efficacy and safety [10], in particular, in keratitis treatment. Purpose: to study the effect of instillations of the colloidal solution of silver nanoparticles measuring 30 nm on the ultrastructure of the corneal epithelium and stroma in rabbits. Material and Methods The study was performed at vivarium of Filatov Institute of Eye Diseases and Tissue Therapy. All experiments followed the bioethical principles stated in the Declaration of Helsinki and the Law of Ukraine On Animal Protection from Cruelty (No 1759-VI dated 15.12.2009) and recommendations of V.D. Mishalova et al. for scientific morphological research [11]. A design of the experiment Groups of the animals studied were: 1) Control group, six intact Chinchilla rabbits which were conjunctivally instilled a normal saline solution; 2) Study group, sixteen Chinchilla rabbits which were conjunctivally instilled a colloidal solution of silver nanoparticles sized 30 nm. Instillations were performed three times a day. The animals were sacrificed by air embolism under thiopental anesthesia at Days 15 and 30 of instillations. Then, eyeball fragments were obtained for morphological examinations. Silver nanoparticles of a spherical shape and 30 nm in size were synthesized by a citrate method at Physics Research Institute of Odessa Mechnikov National University [12]. We used colloidal AgNP solutions with concentration of 0.2 mg/ml. To perform electron microscopy examinations, the corneal tissue samples were fixed in a 2.5% glutaraldehyde solution in phosphate buffered saline (pH 7.4) and postfixed in a 1% osmic acid solution with the same pH of the buffered solution. Afterwards, the samples were dehydrated using a series of increasingly concentrated spirits. The samples were infiltrated and embedded using the Epon-Araldite mixture. Then, the ultrathin sections were contrasted by the method of Reynolds [13]. The sections were examined and imaged using a PEM-100-01 electron microscope. We evaluated ultrastructural changes in epithelial cells of the corneal epithelium, in cells and fibers of the corneal stroma in the central part and in the limbus area. The examinations of the corneal ultrastructure were performed by an Electron Microscopy group of Electron Microscopy and Pathological Morphology Laboratory at Filatov Institute of Eye Diseases and Tissue Therapy. Results and Discussion The ultrastructure of the cornea in the rabbits of Control group The ultrastructure of the cornea in the central part and the limbus area was without pathological changes. Superficial cells of the corneal epithelium had moderate electron-dense cytoplasm in which were placed cisterns of the granular endoplasmic reticulum, numerous free ribosomes, single mitochondria, tonofilaments, a big rod form nucleus (Fig. 1). Single narrow intracellular clefts were detected. Wing cells in the intermediate layer of the corneal epithelium had a typical structure. In the cells, the nucleus was well-defined with sulcate karyolemma; a small electron-transparent rim around the nucleus was noted. Nucleus chromatin was in a dispersed condition. In the cytoplasm, there were diffusely spread a great number of free ribosomes, separated cisterns of the granular endoplasmic reticulum, rare mitochondria. The cells of the superficial and intermediate layers of the corneal epithelium were connected by means of desmosomes and locking plates. Basal cells were placed on the well-defined basal membrane. In the cytoplasm was detected a big nucleous with karyolemma invagination that contained the nucleolus and standard organelles. An electron-dense narrow rim was noted only around the nucleus. In some cells, we detected radiolucency of the intramitochondrial matrix and focal destruction of mitochondrial cristae. The basal membrane of the epithelium, the corneal stroma had no pathological changes (Figure 2).
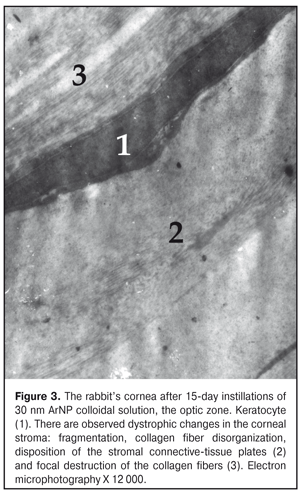
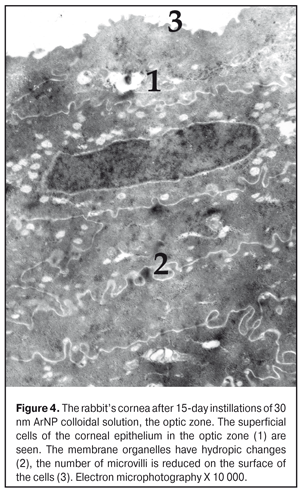
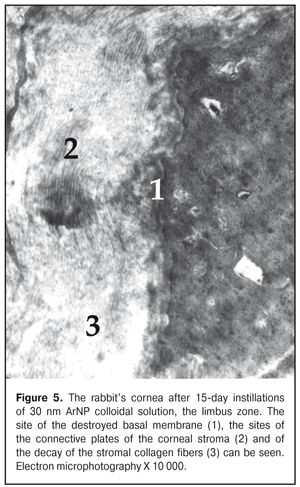
The ultrastructure of the rabbit cornea at Day 15 of AgNP colloidal solution instillations The ultrastructure of the epithelium in the central part of the cornea did not differ much from that in the control animals. However, occasionally, in the cytoplasm of the superficial cells, there were revealed signs of hydropic changes in intracellular membrane structures, especially in mitochondria, where structureless electron-dense foci were also detected (Figure 3). Microvilli in those cells were flat in some places (Fig. 4). The nuclei of a big number of the basal cells had large deep folds and karyolemma invagination, which increases the area of a nucleus surface and characterizes the enhancement of metabolic processes between the nucleus and cytoplasm. In the cytoplasm of some cells, the number of free ribosomes was increased, which gives an evidence of the enhancement of protein synthesis directed towards intracellular needs of the cytoplasm. At the same time, a part of the basal cells had signs of cytoplasm organelle destruction. Areas of destruction were revealed in the structure of the basal membrane (Fig. 5).
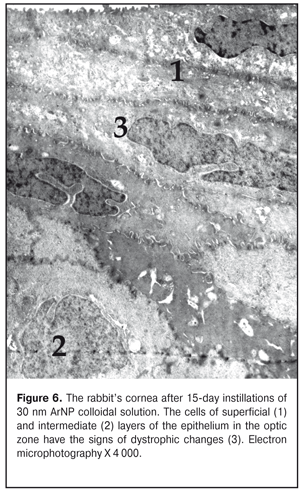
In the subepithelial layers, the structure of the stroma was disorganized; its lammelarity was destroyed. Collagen fibrills were destructed in various degrees. We revealed large areas with their homogenization; occasionally, collagen fibrills were detached. Keratocytes revealed in this area had the increased electron-dense cytoplasm and the nucleus with electron-dense karyoplasms and poorly-defined chromatin. In the cells, there were observed single swollen mitochondria and enlarged cisterns of the granular endoplasmic reticulum. Other organelles were not detectable. In turn, in the limbus area, the superficial cells had well expressed microvilli; the cytoplasmic structures of these cells had no visible pathologic changes apart from the fact that their cytoplasm contained an increased number of free ribosomes. It should be noted that the increased free ribosome level in the cells was noted in all layers of this area of the cornea. At the same time, in the basal cells, compared to control, we noted an increased number of the myelin-like sheaths which are formed in result of membrane structure destruction. Signs of swelling of the main stromal substance as well as structureless electron-transparent foci were noted in the subepithelial area. Alongside the areas with normally structured collagen fibrills, there were areas with separation of fibres, homogenization, or fragmentation of collagen fibril bundles; their electron density was inhomogenous. In the deeper stromal layers, collagen fibrills were preserved in a greater degree. The ultrastructure of the cornea at Day 30 of AgNP colloidal solution instillations The architectonics of the epithelium of the central part of the cornea was preserved and corresponded to that of the intact animals. The ultrastructure of the most superficial cells of the corneal epithelium was without pathologic changes. There were observed cells with the electron-dense cytoplasm. Intercellular contacts were preserved. The ultrastructure of the cells in other layers of the corneal epithelium had no visible structural changes except that their cytoplasm contained myelin-like sheaths. In the subepithelial stromal layers, we revealed areas in which a position of connective-tissue plates was dislocated and an apparent swelling was observed. Keratocytes of the corneal stroma had the nucleus and cytoplasm of the increased electron density, the swollen intramitochondrial matrix with destruction of the cristae, single elements of the granular endoplasmic reticulum with enlarged profiles. There, we also noted the areas of decondensation, fragmentation, and homogenization of the collagen fibrills. Herewith, keratocytes in this area had the pycnotic nucleus, signs of cytoplasm gelization and cytoplasmic organelle destruction, which finally leads to their necrosis (Fig. 6).
In the limbus area, the ultrastructure of the basal cells in the corneal epithelium was characterized by the presence of the areas where cells of various electron density of the cytoplasm were interlaced. We revealed the areas where hemidesmosomes, which connect the basal cells with the basal membrane, were absent or their electron density was reduced. The structure of the wing cells did not differ from that in the control animals. A number of the superficial cells had the structure similar to that of the control animals; the other cells had signs of hydropic changes in the intracellular organelles or their focal destruction. The corneal stroma was not changed, in general. We revealed single foci of swelling of the main corneal stroma substance. The collagen fibrills were detected in different conditions: with normal and loose structure as well as with destructive signs. The ultramicroscopic examination revealed an increase in the number of free ribosomes and an increased number of myelin-like sheaths in the material studied after the 30-day instillations as compared with the 15-day instillations of the silver nanoparticles. The pathologic changes in the material studied, likely, were of compensatory reconstructive character. In this experiment, the silver nanoparticles were not visualized in electron microscopy. So, the studies performed revealed the violations of the membrane organells in the cells of the corneal epithelium; and these violations, and, first of all, of the mitochondria, can be associated with the cytotoxic action of silver nanoparticles. The given results are in agreement with existing data on the possibility of silver nanoparticles to damage the mitochondria [14]. One of the nanosilver cytotoxity mechanisms is a nanoparticle-initiated activation of free radical oxidation in the cytoplasm and the damage to the membrane structure [15, 16]. In our work, we observed the hydropic changes in the organelles of the epithelian cells in the superficial and intermediate layers of the corneal epithelium, the signs of cytoplasmic swelling, which evidences the cytotoxity of silver nanoparticles. Alongside, the increased level of free and bound ribosomes in the epithelial cells and keratocytes gives the evidence of reparative processes in the AgNP-damaged corneal structures. In the corneal stroma, we observed discoupling of the connective-tissue plates, separation of fibres, and fragmentation of the collagen fibrills. It is noticeable that the intensity of fibril alteration decreased with their distance from the epithelium inward the cornea. In our previous investigations, we have observed similar signs of the effect of silver nanoparticles on the collagen fibrills in the inner skin in intradermal administration of the AgNP colloidal solution [17, 18]. Moreover, the previous investigations have demonstrated that the intradermal AgNP injection causes the changes in the functional activity of fibroblasts, the mitotic activity of the epidermic epithelial basal cells, a mast cell degranulation index, a number of macrophage in the dermis. And the possibility of the nanoparticles to increase the functional activity of the fibroplasts rises in line 20 ? 70 ? 30 nm [17, 18]. When modeling bacterial keratitis, the increased neutrophil phagocytic activity rates and the accelerated epithelization of the injured cornea have been revealed [19]. Thus, the conjunctival AgNP instillations causes the dystrophic changes in the separated cells of the corneal epithelium, and the alteration of the stromal collagen fibrills. Herewith, the increased ribosoma level is observed in the epithelial cells, which can explain the accelerated regeneration of the cornea in bacterial keratitis against the AgNP instillations that has been revealed in the previous investigations [19]. Therefore, when assessing the possibility to use AgNP and AgNP-based drugs in the corneal disease therapy, consideration must be given both to their antimicrobial, anti-inflammatory properties and to their cytotoxity. Conclusions
Longlasting three time daily instillations of the AgNP colloidal solution into the conjuctival cavity in the dose of 30 nm and with the duration of 15 and 30 days causes the ultrastructural changes in the cells of the anterior corneal epithelium, the basal membrane, the corneal stromal cells and fibres in the rabbits. In all cells of the corneal epithelium, the increased free ribosome level and the decreased number of cristae in the mitochondria were observed. In the basal cells, we revealed the increased number of the myelin-like sheaths reflecting the membrane structure decay processes, this was especially apparent in the material after the 30-day AgNP instillations. The dystrophic changes in the keratocytes, destruction of the connective-tissue plates, separation of the fibers and collagen fibril fragmentation were revealed in the corneal stroma. References
|